Abstract
Purpose. The study was undertaken to identify pre-treatment clinical and histopathological factors of importance for response and survival after cisplatin-based combination chemotherapy, in patients with locally advanced or metastatic transitional cell carcinoma of the urothelium. Patients and methods. Clinical, laboratory and histopathological data from 178 consecutive patients, representing all patients treated between 1991 and 2001 in a single institution, were collected. Correlations between these data and response and survival after chemotherapy were analysed using univariate and multivariate analyses. Results. Absence of visceral metastasis was the only parameter with independent correlation to the response to chemotherapy. Two of the analysed parameters were independently associated with increased survival: good performance status (PS≤1) and absence of visceral metastases. Stratification of the patient material according to number of these risk-factors present showed strong association with survival. Conclusion. It was possible to predict survival from pre-treatment clinical parameters and consequently it is possible to select groups with a high and low probability of obtaining long term survival following cisplatin-containing chemotherapy.
Despite radical surgical and radiotherapeutic treatments offering excellent results in terms of local control, 20–40% of patients diagnosed with invasive bladder cancer will eventually develop metastasis and succumb to the disease. These patients have a median life expectancy between three and six months if left untreated. There is little doubt that the advancement of chemotherapy has improved the life expectancy of these patients over the past decades. A range of chemotherapeutic drugs have been tested against transitional cell carcinoma, however cisplatin is still considered the single most efficient drug in treatment of these tumours used alone Citation[1] or in combination Citation[2]. Although direct evidence or quantification of the survival benefit from chemotherapy vs. best supportive care has never been investigated in prospective studies, indirect evidence can be inferred from the survival benefit demonstrated by multidrug chemotherapy over single drug treatment Citation[3]. Various combination regimens with cisplatin have prolonged the survival of patients with advanced bladder cancer to between 13 and 24 months Citation[4], Citation[5], and response rates above 75% Citation[4] have emphasized that bladder cancer must be considered a chemotherapy sensitive disease. Although chemotherapy trials have demonstrated long-term survival in a small proportion (up to 5–6%) of patients, this treatment modality is still considered palliative.
Cisplatin containing combination chemotherapy in bladder cancer is toxic treatment with significant side effects. Its use should preferably be warranted by a high probability of a survival benefit in the individual patient. Demonstration of significant predictive factors for response and survival will facilitate and optimise selection of patients in this respect.
In order to investigate the predictive power of commonly available clinical and histopathological parameters for the outcome of chemotherapy and survival, we have collected and analysed data from all patients receiving platinum based chemotherapy for transitional cell carcinoma at our institution over a 10-year period from 1991 to 2001. Previous studies have indicated that pre-treatment prognostic information on survival can be gained from performance status score, presence of visceral metastases and elevated serum levels of alkaline phosphatase Citation[5–10]. Similarly response to chemotherapy is associated with the presence of visceral metastases, level of haemoglobin and performance status Citation[8], Citation[9].
Material and methods
Patients
The study included a total of 178 consecutive patients receiving first line platinum containing combination chemotherapy for locally advanced (T4B, N2-3) and/or metastatic (M1) transitional cell carcinoma (TCC) at the Department of Oncology, Aarhus University Hospital, Denmark, in the 10-year period between 1991 and 2001. All patients had transitional cell carcinoma of the urothelial tract (urethra, bladder, ureters or renal pelvis) histologically diagnosed or confirmed at our institution. All patients received one of seven cisplatin-based combination chemotherapy schedules as shown in and were included in either one of four phase II protocols, one phase III protocol or were treated with the standard regimen at the relevant time (MVAC or GC). Patients were treated to a maximum of 6 cycles unless progression or unacceptable toxicity appeared. Patients receiving standard treatment were prospectively followed under the same conditions as in the protocols.
Table I. Chemotherapy regimes 1991–2001.
Patients presenting with locally advanced disease, obtaining a significant partial or complete response to chemotherapy, were offered consolidating surgery or, more often, radiotherapy when applicable.
Methods
Diagnostic work-up and staging of patients prior to treatment included CT scans of thorax, abdomen and pelvis, bone scintigraphy in case of bone pain or other skeletal symptoms and ultrasound with fine needle aspiration of lesions unresolved by CT scan. All patients were followed with CT scans every 3rd month until progression.
Patient files, laboratory and radiographic data were retrieved from archives. Patient characteristics, laboratory test results, staging procedures and the histopathology of the tumour prior to chemotherapy were recorded. Continuous numerical data were dichotomised, using either median values (age) or local laboratory normal limits (, first column) as cut-off points. Visceral metastases were defined as metastases outside the urothelial tract, the bladder bed and the lymphatic system. Histopathology data included tumour grade (≤ 3 vs. 4) Citation[11] and tumour type (pure TCC vs. TCC with squamous or adenomatous differentiation). Pre-treatment TNM Citation[12] status for each patient was re-evaluated using reports from the urologic surgeons, pathology reports and reports from diagnostic imaging (x-ray, CT scans, ultrasonography and bone scintigraphy).
Table II. Prognostic factors for survival (univariate analyses)
Response to chemotherapy was graded using the WHO-criteria Citation[13] into complete response (CR), partial response (PR), no change (NC) and progressive disease (PD). This was done after every two or three cycles of chemotherapy according to the trial protocols and finally four weeks after the last cycle. Most data were collected prospectively as patients were treated in research protocols ().
Statistics
Fifteen clinical, histopathological and laboratory parameters were tested for their association with response and survival after chemotherapy. Survival time was calculated from the start of chemotherapy until death or censoring (September 2006) giving a follow up period between 5 and 15 years. All 178 patients were included in the survival analysis. Parameters were compared in univariate analyses using log rank test. Survival distributions were estimated using the Kaplan-Meier method Citation[14]. Variables with p-values equal to or below 0.1 in univariate analyses were included in multivariate analyses using Cox regression model. P-values less than 0.05 (two-sided test) were considered significant.
The relationship between clinical parameters and the response to chemotherapy was examined in univariate analyses using χ2-test. Variables with p-values equal to or below 0.1 in univariate analyses were included in multivariate analyses using a binary logistic regression model. P-values less than 0.05 (two-sided test) were considered significant.
All statistical analyses were performed using the SPSS 13.0 statistical package (SPSS Inc., IL, USA)
Results
The investigated clinical, histopathological and laboratory data of potential prognostic importance are listed in along with results of the univariate analyses of their association with response to and survival after chemotherapy.
Male/female ratio was 4:1 (141 males of 178, 79%), and the median age for the cohort of patients was 59 years (range 36 to 75 years) at the initiation of chemotherapy. Median performance status score was 1, and 140 patients (79%) had a score of 0 or 1.
A large group of patients had subnormal haemoglobin at presentation (96 of 178, 54%), whereas only a minority showed reduced renal function. Thus 13% (23 of 178) had elevated S-creatinine level and 9% (16 of 177) a GFR below 60 ml/min. Nine percent of the patients had elevated ALAT (16 of 177), 14% (25 of 177) had elevated LDH while one third of the patients showed increased S-alkaline phosphatase level (58 of 177, 33%).
Approximately half of the patients (89 of 176, 51%) were treated on the basis of locally advanced primary disease stage (T4B and/or N>1) whereas the other half (87 of 176, 49%) had remote metastatic (M1) disease at the start of therapy. The number of patients with metastatic spread to the lungs, liver, or bone was similar. Nineteen percent (34 of 177) had lung metastasis, 20% (34 of 169) had liver metastasis and 19% (33 of 178) had bone metastases.
Number of missing laboratory data and staging procedures are shown in the brackets in .
Predictive factors for response
One hundred and twenty-five patients were evaluable for response to chemotherapy. Of these, 22 patients (18%) had a CR and 35 patients (28%) a PR for an overall response rate of 46%. Fifty patients (40%) had no change and 18 patients (14%) showed progressive disease as best response (). Univariate analyses demonstrated performance status, S-LDH and visceral metastases to be statistically significantly related to response (). Of patients in good performance status (PS≤ 1) 53% responded to the treatment while only 23% of the patients with a low performance score (PS> 1) achieved a response. The majority of patients had normal levels of S-LDH and 50% of these patients responded to the treatment, whereas only 26% of the patients with elevated S-LDH obtained response. Patients with visceral metastasis demonstrated a response rate of 33% compared to 65% in patients without such organ involvement. In univariate analyses elevated S-alkaline phosphatase, presence of liver metastases and bone metastases all showed p-values below 0.1 and these parameters were included in the multivariate regression analysis. This analysis established that presence or absence of visceral metastasis was the only independent predictive factor for response in the present material (p=0.04) ().
Table III. Predictive factors for response (multivariate analysis)
Prognostic factors for survival
The overall median survival was 12.1 months (95% CI: 10.52–13.67) with a 9.6% censoring rate at a follow-up time of minimum 61 months. The five-year survival rate was 11.2%. Univariate analyses demonstrated performance status, B-haemoglobin, GFR, S-LDH, S-alkaline phosphatase, visceral metastases and presence vs. absence of lung metastases, liver metastases and bone metastases () to be significantly related to survival. Cox’ multivariate regression analysis showed that performance status (PS≤1 vs. PS≥2) and visceral metastasis (absent vs. present) were independent prognostic factors for survival ().
Table IV. Prognostic factors for survival (multivariate analysis)
For patients with PS≤1, the median survival was 14.8 months (95% CI: 12.98–16.62) vs. 5.2 months (95% CI: 3.37–7.10 months) in patients with PS≥2. Patients with visceral metastases shoved a median survival of 8.5 months (95% CI: 6.97–9.96) vs. 18.0 months (95% CI 15.19–20.88) in patients with only bladder involvement or nodal spread.
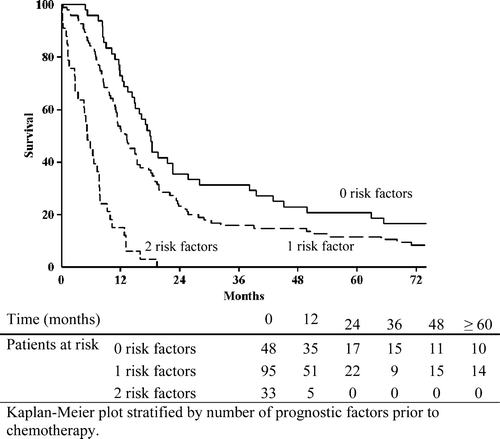
Risk groups
Based on the result of the Cox regression model patients were retrospectively divided into three groups, according to the number of independent prognostic factors they presented with prior to chemotherapy (). The group of patients with no risk factors contained 48 patients (27%). The median survival in this group was 18 months (95% CI: 15.58–20.49) and the five-year survival was 21% (). The groups of patients having one or two risk factors encompassed 95 (54%) and 33 (19%) patients respectively. Patients with one risk factor had a median survival time of 13.2 months (95% CI: 10.13–16.33) and patients with two risk factors had a median survival time of 5.2 months (95% CI: 3.28–7.18). As seen in this difference was highly significant (p<0.0001). All long term survivors (> 60 months) were found among patients with 0 or 1 risk factors ().
Table V. Risk factors influence on survival
Separating the patients according to the number of adverse prognostic factors was also found to predict response to chemotherapy. Amongst patients with no adverse prognostic factors a response rate of 63% (12 of 19) was seen, whereas patients with one or two adverse prognostic factors responded in 53 and 18% of cases respectively (). This association was highly significant in univariate analysis (p=0.002).
Table VI. Risk factors influence on response
Discussion
Locally advanced or metastatic transitional cell carcinoma is, based on the relative high response rates (39–78%) Citation[4], Citation[8] obtained with systemic combination chemotherapy, a chemotherapy sensitive disease. The long-term survival is, however, still poor and chemotherapy is curative in only a very limited number of patients.
The results of this study confirm previous studies. In 1992 Loehrer et al. Citation[8] found that performance status, absence of visceral metastasis and no weight loss was associated with improved survival and response to chemotherapy. Bajorin Citation[6] and colleagues from the Memorial Sloan-Kettering Cancer Centre and later Bellmunt Citation[7] and the Spanish Oncology Genitourinary Group found that visceral metastasis and performance status had profound impact on survival. A phase II study by Stadler et al. Citation[5] showed that visceral metastasis was the only prognostic parameter for survival, whereas Sengelov et al. Citation[9] found that survival was influenced by performance status, bone and liver metastasis, alkaline phosphatase and s-creatinine. Thus, presence of visceral metastasis and low performance status have, both in this study and in earlier clinical trials, been shown to be adverse prognostic factors for survival in patients with advanced or metastatic transitional cell carcinoma. The most likely explanation is that these parameters reflect an increased tumour burden and advanced cancer disease state. Presence of only locally advanced disease status or unresectable local relapse without recognizable systemic spread indicates relative lack of tumour aggressiveness and metastatic potential. The observed prolonged survival in these patients might well, at least in part, be a consequence of a more indolent nature of these tumours per se along with a product of a beneficial effect of chemotherapy.
Sengelov Citation[9] found that response to chemotherapy was influenced by performance status, visceral metastasis and low haemoglobin, whereas Loehrer et al. Citation[8] found that response was influenced by performance status, weight loss and visceral metastases. The present study found presence of visceral metastases to be the only independent predictive factor for response to chemotherapy. The reason why TCC seems to responds better to chemotherapy in T and N sites than in visceral metastatic sites is not clear, but could well be a consequence of inherent biologic differences between tumours with and without metastatic propensity as mentioned above.
Since only a minority of the chemotherapy administered to patients with metastatic bladder cancer results in long term survival – in the present study just above 10%-prolongation of survival and palliation of symptoms remain the major goals for the vast majority of patients treated. Selection of patients for chemotherapy, and omitting toxic and potentially harmful treatment to patients unlikely to benefit is thus a key challenge. Thorough review of the available literature and prospective analyses of the actual chemotherapy administered has been the aim of a large coherent series of publications from Sweden Citation[20], Citation[21]. They concluded that although with cisplatin based multidrug chemotherapy response rates were high, the lack of randomised comparisons with best supportive care precludes definite conclusions regarding survival benefit gained. The differences observed in median survival between less intensive regimes (4.5 months with metotrexate/vinblastine Citation[22]) and recent, more intensive chemotherapy (up to 13–15 months with cisplatin based regimes Citation[2]) however strongly suggests a survival benefit in selected patient groups. The basis on which to select patients for chemotherapy is however undocumented. Phase II protocols are often restricted to patients in relative good general condition without significant comorbidity. Data derived from such studies are consequently biased compared to the patient populations of everyday practise. The present study comprises data from consecutive patients – thus a patient population representative of the full clinical spectrum of patients requiring treatment.
The paucity of sufficiently large, prospective trials to provide a secure evidence base for these routine decisions stresses the need for initiation and design of protocols to answer these questions and constitute the justification for retrospective analyses like the present.
In our study stratification according to risk factors defines a group of patients with relatively good prognosis (nodal and local disease only) and a group with a very short expected survival (visceral metastatic disease and/or low performance status). Although it can not be concluded from these data that patients in the latter group, “bad prognosis patients”, do not obtain a survival benefit from chemotherapy, less than 40% of the patients in our study survive to the end of planned chemotherapy (6 months). The toxicity of relevant chemotherapy regimes is significant; its influences on immediate quality of life are, as mentioned above, not well documented. This, along with the fact that response rates are found to be significantly lower (only 18 vs. 63% and 53% with zero and one risk factor) warrants cautious consideration and discussion with patients in this group regarding other, more immediate means of symptom palliation.
Finally, stratification according to known prognostic factors is important in future randomised clinical trials. Selecting patients with high probability of responding and minimising the differences in patient populations reduces the risk of selection bias making differences attributable to differences in efficacy of the examined therapeutic combinations more evident.
Acknowledgements
Declaration of interest: The authors report no conflict of interest. The authors are responsible for the content and writing of the paper.
References
- Roth BJ. Chemotherapy for advanced bladder cancer. Semin Oncol 1996; 23: 633–44
- von der Maase H. Current and future perspectives in advanced bladder cancer: Is there a new standard?. Semin Oncol 2002; 29(1 Suppl 3)3–14
- Lohrer PJ, Einhorn LH, Elson P, et al. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine and doxorubicin in patients with metastatic urothelial carcinome: A cooperative group study. J Clin Oncol 1992; 10: 1066–73
- Bellmunt J, Guillem V, Paz-Ares L, Gonzalez-Larriba JL, Carles J, Batiste-Alentorn E, et al. Phase I-II study of paclitaxel, cisplatin, and gemcitabine in advanced transitional-cell carcinoma of the urothelium. Spanish Oncology Genitourinary Group. J Clin Oncol 2000; 18: 3247–55
- Stadler WM, Annamaria H, von der Maase H, Debasish R, Dogliotti L, Seymour L, et al. Long-term survival in phase II trials of gemcitabie plus cisplatin for advanced trasitional cell cancer. Urol Oncol 2002; 7: 153–7
- Bajorin DF, Dodd PM, Mazumdar M, Fazzari M, McCaffrey JA, Scher HI, et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol 1999; 17: 3173–81
- Bellmunt J, Albanell J, Paz-Ares L, Climent MA, Gonzalez-Larriba JL, Carles J, et al. Pretreatment prognostic factors for survival in patients with advanced urothelial tumors treated in a phase I/II trial with paclitaxel, cisplatin, and gemcitabine. Cancer 2002; 95: 751–7
- Loehrer PJ, Sr, Einhorn LH, Elson PJ, Crawford ED, Kuebler P, Tannock I, et al. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: A cooperative group study. J Clin Oncol 1992; 10: 1066–73
- Sengelov L, Kamby C, Geertsen P, Andersen LJ, von der Maase H. Predictive factors of response to cisplatin-based chemotherapy and the relation of response to survival in patients with metastatic urothelial cancer. Cancer Chemother Pharmacol 2000; 46: 357–64
- Sengelov L, Kamby C, von der Maase H. Metastatic urothelial cancer: Evaluation of prognostic factors and change in prognosis during the last twenty years. Eur Urol 2001; 39: 634–42
- Bergkvist A, Ljungqvist A, Moberger G. Classification of bladder tumours based on the cellular pattern. Preliminary report of a clinical-pathological study of 300 cases with a minimum follow-up of eight years. Acta Chir Scand 1965; 130: 371–8
- Sobin LH, Fleming ID. TNM classification of malignant tumors. 5th ed. Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer 1997; 80: 1803–4
- World Health Organization. WHO handbook for reporting results of cancer treatment. 48th ed. Geneva: WHO offset publication; 1979.
- Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–81
- Sengelov L, Nielsen OS, Kamby C, von der Maase H. Platinum analogue combination chemotherapy: cisplatin, carboplatin, and methotrexate in patients with metastatic urothelial tract tumors. A phase II trial with evaluation of prognostic factors. Cancer 1995; 76: 1797–803
- Andersen LJ, Sengelov L, Kamby C, von der Maase H. Cisplatin, methotrexate and mitoxantrone in patients with metastatic or advanced urothelial cancer. Acta Oncol 1998; 37: 110–2
- von der Maase H, Andersen L, Crino L, Weinknecht S, Dogliotti L. Weekly gemcitabine and cisplatin combination therapy in patients with transitional cell carcinoma of the urothelium: A phase II clinical trial. Ann Oncol 1999; 10: 1461–5
- von der Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: Results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 2000; 18: 3068–77
- von der Maase H, Geertsen PF, Baekke J, Daugaard G, Sengelov L, Pedersen D, et al. Gemcitabin, cisplatin and paclitaxel (GCP) as first-line treatment of locally advanced and metastatic cell carcinoma (TCC) of the urothelium. American Society of Clinical Oncology. Thirty-Ninth Annual Meeting 2003; 22: 410
- Nilsson S, Ragnhammer P, Nygren P, Glimelius B. A systematic overview of chemotherapy effects in Urothelial bladder cancer. Acta Oncol 2001; 40: 371–90
- Ragnhammer P, Brorsson B, Nygren P, Glimelius B. A prospective study of the use of chemotherapy in Sweden and and assessment of the use in relation to scientific evidence. Acta Oncol 2001; 40: 391–411
- Mead GM, Russell M, Clark P, et al. A randomized trial comparing methotrexate and vinblastine (MV) with cisplatin, methotrexate and vinblastine (CMV) in advanced transitional cell carcinoma: Results and a report on prognostic factors in a medical research council study. Br J Cancer 1998; 78: 1967–75