Abstract
Background. The aim of this exploratory study was to evaluate whether significant differences exist between whole blood selenium levels (WBSL) in patients with prostate cancer (PC), benign prostatic hyperplasia (BPH), healthy male inhabitants (HMI) in northern Bavaria and the normal value. Furthermore, we investigated whether differences exist between prostatic tissue selenium levels (PTSL) in patients with PC, BPH and the benign tissue surrounding the PC. Material and methods. We prospectively evaluated WBSL in 24 patients with PC, 21 patients with BPH, and 21 HMI. Measurements of PTSL were performed in 17 patients with PC and 22 patients with BPH. In 9 cases with PC, measurements were also done in the benign tissue surrounding the carcinoma. Measurements were performed using automated graphite furnace atomic absorption spectrophotometry. Results. In patients with PC, there is a significantly lower WBSL in comparison to HMI (p=0.04). There is no significant difference in WBSL between BPH-patients and HMI (p=0.13) and between PC- and BPH-patients (p=0.67). In all patients and the HMI, there is a significantly lower WBSL in comparison to the recommended normal value of 85 – 162 µg/l (p<0.01). There is no significant difference in PTSL between PC and BPH (p=0.49), and between PC and the tissue compartment surrounding the PC (p=0.56). PTSL seemed to be reduced in the compartment surrounding the PC in comparison to BPH (p=0.03). In PC-patients, there is no significant correlation between WBSL and prostate specific antigen (PSA) (?=−0.20; p=0.36), Gleason score (?=0.32, p=0.13), and T-stage (?=0.22; p=0.23). Conclusion. Since the WBSL measured in all men with PC and BPH, and in HMI participating in our study were significantly lower than the recommended normal range, our findings may support the recommendation of selenium supplementation.
Highly reactive, oxygen-derived radicals participate in human physiology and pathology. The number of physiologically generated oxygen radicals is reduced by different enzymatic and non-enzymatic antioxidants. The main enzymatic antioxidants include superoxide dismutase, catalase and glutathione peroxidase. A well known and important non-enzymatic cofactor is the trace element selenium. Physiologically, there is a finely balanced homeostasis of free oxygen radicals. A quantitative imbalance between oxidative and antioxidative capacities is believed to contribute to a broad variety of diseases including cancer. Various epidemiological studies have shown an association between low selenium levels and an increased risk of cancer incidence Citation[1–13].
There is a dramatic international variation in prostate cancer (PC) mortality rates. Biomarkers, including testosterone and insulin-like growth factor, and nutritional factors, especially meat, fat and dairy intake, have been linked to a greater risk of disease. Furthermore, Germany is a region with low environmental selenium soil levels because of long-term intensive agriculture. Several studies have shown reduced blood, serum and plasma levels of selenium in patients with PC Citation[14–18].
Higher consumption of selenium and vitamin E, fructose/fruits and tomatoes have all been associated with a reduced occurrence of PC, but as yet their efficacy for prevention remains unproven Citation[19–22]. In 1996, the Nutritional Prevention of Cancer Study underscored the possible protective role of selenium for PC. This multicenter, double-blind, randomized placebo-controlled trial was designed to test whether selenium supplementation could prevent skin cancer. A total of 1 312 subjects with a previous history of skin cancer were randomized to 200 µg selenium daily or a placebo and treated for a mean time of 4.5 years. After a total follow-up averaging 6.4 years, no difference in skin cancer was found between the selenium and placebo groups. However, a 50% reduction in overall cancer mortality was seen in the selenium group. Furthermore, the number of men diagnosed with PC was 67% lower in the selenium group compared with the controls. This study has generated considerable excitement about the possible cytoprotective role of selenium in PC Citation[23], Citation[24]. A recently published meta-analysis has shown that selenium administration in men with low plasma selenium levels reduced the PC risk Citation[25]. New international studies which started recently to investigate a possible preventive effect on PC by selenium supplementation are the European study PRECISE and the North American study SELECT. In the PRECISE trial (Prevention of Cancer by Intervention with Selenium), 33 000 Europeans from Great Britain, Sweden and Denmark are to be recruited Citation[11]. For the SELECT trial (Selenium and Vitamin E Cancer Prevention Trial), which investigates the influence of supplementation of selenium methionine and vitamin E on the incidence of PC, 35 534 men from the USA, Canada and Puerto Rico were recruited from 2001 to 2004. After a follow-up of 7 further years, first results will be published in 2011 Citation[26], Citation[27].
Published data about blood selenium levels in patients with BPH and selenium tissue levels in patients with PC and BPH are scarce Citation[18], Citation[28–30].
The aim of this exploratory study was to evaluate whether significant differences exist between whole blood selenium levels in patients with PC, BPH, HMI and the normal value. The study was also motivated by the numerous questions regarding selenium supplementation as a measure which could possibly help to prevent PC, which have been asked, in particular, by HMI from the region within the context of uro-oncological check-ups. Since hardly any data are available on selenium levels in the blood of patients with BPH, we also measured the WBSL of these patients. To obtain a better comparison, we then measured, in addition, WBSL in HMI of the same geographical region (northern Bavaria) from which the patients were drawn. This was necessary in order to be able to take known regional – in part diet-related – variations of whole blood selenium values as compared to the normal range of values sufficiently into account. In addition, we studied PTSL in patients with PC and BPH. Since for ethical reasons it was not possible to subject HMI to a prostate biopsy. Since we assume differences between the selenium levels in carcinoma tissue and the tissue surrounding the carcinoma, we also determined PTSL in the tissue compartment surrounding the PC.
Methods
At the end of 2003, we started the prospective pilot study. In 46 consecutive patients, total prostate volume and transition zone volume were measured, and all patients underwent transrectal ultrasound-guided octant biopsy of the prostate. It is known that differentiation between malignant and benign prostatic tissue by ultrasound is not useful and has a very low predictive value. Therefore, we accepted only the histological result. According to histological results of prostate biopsy, patients were divided into two groups: a PC-group and a BPH-group. 24 patients were identified as having PC and 22 patients as having BPH. In nine patients with PC, biopsies were histologically differentiated in malignant and benign compartments of tissue by the pathologist. In this small patient number, we studied whether there are differences in PTSL between malignant and benign tissue compartments.
We prospectively evaluated WBSL in all patients with PC, 21 patients with BPH, and 21 HMI. The patients as well as the HMI were inhabitants of northern Bavaria. Measurements of PTSL were performed in 17 patients with PC and in all patients with BPH. In nine cases with PC, measurements were performed in histological benign tissue surrounding the carcinoma. Measurements were taken using automated graphite furnace atomic absorption spectrophotometry. For the comparison of WBSL, we used the recommended normal range of 85 – 162 µg/l Citation[31]. This normal range comes from the University of Jena (Thuringia), and we can use it for the entirety of Germany, because we believe there is not a substantial variation of diet among German people. Since this range is mean±1.96*std, we conclude mean=123.5 and std=19.6.
Statistical analysis
For processing and statistical analysis of all data, the SPSS/PC Software package, version 15.0 (SPSS GmbH, Munich, Germany), was used. Descriptive statistics were computed for continuous variables. The statistics computed included mean, median, minimum, maximum and standard deviations of continuous variables. Because of the small number of subjects and the low power of a test on normality, comparisons between groups were done by using the nonparametric Mann-Whitney U test by ranks, and correlations were computed by using Spearman′s rank correlation coefficient. An exception was made for the comparison with recommended normal range in . Since only mean and standard deviation are known and individual values are unknown, the use of independent t-test was necessary.
Figure 1. White blood selenium levels with standard deviations in patients with PC, BPH and in healthy inhabitants
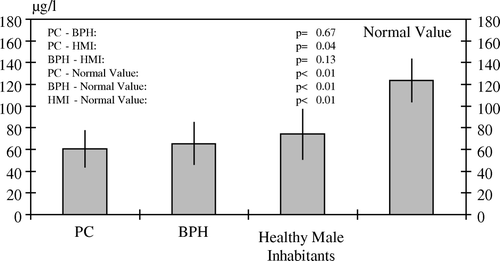
All p-values resulted from two-sided statistical tests; values of p<0.05 were considered to be statistically significant.
Results
In PC-patients with a mean age of 69.1±9.63 years (median 67.5; range 52–87), we measured a mean WBSL 60.1±17.2 µg/l (median 57.9; range 29.1–98.5) compared to BPH-patients with a mean age of 63.7±10.8 years (median 63.0; range 40–83) with 64.1±19.1 µg/l (median 58.7; range 42.7–123) and HMI with a mean age of 48.1±9.80 (median 47.0; range 31–69) with 74.1±23.0 µg/l (median 74.0; range 43.6–127). In patients with PC, there is a significantly lower whole blood selenium level in comparison to the HMI (p=0.04). There is no significant difference in WBSL between patients with BPH and HMI (p=0.13) and between patients with PC and patients with BPH (p=0.67). In all patients as well as in the HMI, there is a significantly lower whole blood selenium level in comparison to the recommended normal value of 85–162 µg/l (p<0.01) ().
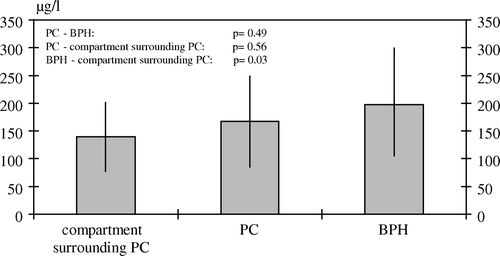
In PC-tissue, we measured a mean selenium level of 167±81.5 µg/l (median 179, range 55.6–290), in BPH-tissue 198±92.3 µg/l (median 165, range 89.6–461) and in the benign tissue surrounding the carcinoma 139±61.5 µg/l (median 132; range 46.5–281). There is no significant difference in PTSL between PC and BPH (p=0.49), and between PC and the tissue compartment surrounding the PC (p=0.56). PTSL seemed to be reduced in the tissue compartment surrounding the PC in comparison to BPH (p=0.03) ().
Figure 2. Tissue selenium levels with standard deviations of PC, BPH and the compartment surrounding PC
In patients with PC, there is no significant correlation between WBSL and PSA (?=−0.20, p=0.36), Gleason score (?=0.32, p=0.13), and T-stage (?=0.22, p=0.23). A detailed summary of characteristics of patients with prostate cancer is presented in .
Table I. Characteristics and whole blood selenium levels of patients with prostate cancer.
Discussion
Present data confirm other published information with reduced blood, serum and plasma levels of selenium in patients with PC Citation[14–18]. They may serve as further evidence for a quantitative imbalance between oxidative and antioxidative capacities in patients with PC. An imbalance between oxidant and antioxidant capacities can either be the result of excessively high radical production or insufficient defense mechanisms.
Men with lower serum selenium levels at an average of 3.8 years before diagnosis had a 4 to 5-fold increased risk of PC compared to men with higher selenium levels Citation[15]. The way in which selenium could suppress neoplasia is not clear at all. In vitro, selenium compounds produce direct anti-tumour effects, as demonstrated by tumour cell growth inhibition and apoptosis promotion Citation[32]. A large portion of selenium is incorporated into glutathione peroxidase, which is known to reduce lipid peroxide. Human PC is known to lose expression of glutathione-S-transferase almost universally. This loss of expression occurs in the earliest stages of PC, including precursor lesion prostatic intraepithelial neoplasia. The glutathione-S-transferase is involved in the reduction of lipid peroxide. It is possible that glutathion peroxidase could compensate for the loss of glutathione-S-transferase in the prostate, and a threshold of selenium is necessary to maintain adequate glutathion peroxidase activity Citation[33–35]. Selenium is known to participate in other cellular functions beyond glutathione peroxidase, including suppressing the growth of PC-cells in vitro, and at high levels can be cytotoxic Citation[36]. In vitro selenium was able to inhibit the growth and to induce the apoptosis of PC-cells compared to normal prostate cells. Androgen-responsive cells were the most sensitive to selenium growth suppression Citation[37].
Our data also confirm reduced blood levels of selenium in patients with BPH Citation[18]. However, whether there is a causal connection between the development of this disease and the low WBSL remains speculation. No data could be found on this question in the relevant literature.
Concerning the PTSL of patients with BPH and PC, only very limited data is available in the literature. In contrast to our data, one study found lower values rather in patients with BPH compared to patients with PC, while the PTSL of healthy men were found to be approximately at the same level as those of patients with BPH Citation[18], Citation[30]. The difference to our tissue results cannot be exhaustively explained, since further literature data which could be used for comparison is not available. An explanation from a statistical point of view is, in our opinion, not conclusive, since the working team of Zachara et al. carried out tissue measurements in no more than 32 patients with PC and 40 patients with BPH.
The significantly lower PTSL in the tissue compartment surrounding the PC compared to BPH tissue found in our analysis are difficult to explain at present; they might suggest a significantly increased consumption of selenium in the prostate tissue within the scope of defense processes against PC. This might be taken as an indirect indication in favor of selenium supplementation for the purpose of PC prevention. Two studies showed that oral administration of selenium, in comparison to controls, lead to a significant increase in the PTSL Citation[28], Citation[29], but not in the seminal vesicles Citation[29].
A correlation between WBSL in patients with PC and the parameters T-stage, PSA and Gleason score could not be shown in our analysis, which is probably due, in the first place, to the lower number of patients.
The fact that the WBSL in HMI is significantly below the recommended normal range is an additional indication of a possible selenium deficiency in the population of northern Bavaria, which suggests the recommendation of selenium supplementation.
Conclusion
In particular the significantly low WBSL in patients with PC and BPH as well as in HMI of northern Bavaria compared to the normal range corroborate our recommendation for selenium supplementation. Low selenium levels are likely to be related to PC and this suggests that selenium might be useful in the prevention of PC. New international studies (PRECISE and SELECT) which started recently to investigate a possible preventive effect on PC by selenium supplementation have to give the answer.
Acknowledgements
The authors would like to extend special thanks to the company Biosyn (Fellbach, Germany) for supporting the study and measuring whole blood and tissue selenium levels.
References
- Buentzel J, Glatzel M, Kisters K, Muecke R, Bruns F, Schoenekaes K, et al. Selenium as radioprotector in head and neck cancer patients – first clinical results. Trace Elem Electrolytes 2006; 23: 178–80
- Buentzel J, Glatzel M, Muecke R, Bruns F, Kisters K, Micke O. Scavenging of free radicals and late toxicity of radiochemotherapy in head and neck cancer patients. Trace Elem Electrolytes 2006; 23: 178–80
- Buentzel J, Micke O, Glatzel M, Froehlich D, Bruns F, Muecke R, et al. Serum selenium in head and neck cancer patients – a new marker of tumor activity?. Anticancer Res 2005; 25: 1711–2
- Funke AM. Potential of selenium in gynecological oncology. Med Klin 1999; 94: 42–4
- Knekt P, Aromaa A, Mantela J. Serum selenium and subsequent risk of cancer among Finnish men and woman. J Natl Cancer Inst 1990; 82: 864–8
- Micke O, Buentzel J, Bruns F, et al. Selenium in oncology – Past, presence and future. Trace Elem Electrolytes 2006; 22: 66
- Muecke R, Micke O, Schoenekaes KG. Serum selenium levels, glutathione peroxidase activities and serum redox potential levels in patients with untreated non-small cell lung cancer and adenocarcinoma of the rectum. Trace Elem Electrolytes 2000; 17: 119–23
- Muecke R, Glatzel M, Reichl B, Bernd-Skorka R, Buentzel J, Bruns F, et al. Sodium selenite in gynecologic radiation oncology – results of a prospective randomized observation study. Trace Elem Electrolytes 2006; 23: 173–7
- Pawlowicz Z, Zachara BA, Trafikowska U, Maciag A, Marchaluk E, Nowicki A. Blood selenium concentrations and glutathione peroxidase activities in patients with breast cancer and with advanced gastrointestinal cancer. J Trace Elem Electrolytes Health Dis 1991; 5: 272–7
- Pawlowicz Z, Zachara BA, Trafikowska U, Nowicki A. Low levels of selenium and activity of glutathione peroxidase in blood of patients with gastrointestinal neoplasma. Pol Tyg Lek 1993; 48: 554–6
- Rayman MP. The importance of selenium to human health. Lancet 2000; 356: 233–41
- Torun M, Aldemir H, Yardim S. Serum selenium levels in various cancer types. Trace Elem Electrolytes 1995; 12: 186–90
- Turan B, Delilbasi E. The serum selenium and immunoglobulin levels in healthy and cancer cases. J Biochem 1992; 17: 29–36
- Brinkmann M, Reulen RC, Kellen E, Buntinx F, Zeegers MP. Are men with low selenium levels at increased risk of prostate cancer. Eur J Cancer 2006; 42: 2463–71
- Brooks JD, Metter EJ, Chan DW. Plasma selenium level before diagnosis and the risk of prostate cancer development. J Urolog 2001; 166: 2034–8
- Li H, Stampfer MJ, Giovannuvcci EL, Morris JS, Willet WC, Gaziano JM, et al. A prospective study of plasma selenium levels and prostate cancer risk. J Natl Cancer Inst 2004; 96: 696–703
- Ozmen H, Erulas FA, Karatas F, Cukurovali A, Yalcin O. Comparison of the concentration of trace metals (Ni, Zn, Co, Cu and Se), Fe, vitamins A, C and E, and lipid peroxidation in patients with prostate cancer. Clin Chem Lab Med 2006; 44: 175–9
- Zachara BA, Szewczyk-Golec K, Tyloch J, Wolski Z, Szylberg T, Stepien S, et al. Blood and tissue selenium concentration and glutathione peroxidase activities in patients with prostate cancer and benign prostate hyperplasia. Neoplasma 2005; 52: 248–54
- Brawley OW, Parnes H. Prostate cancer prevention trials in the USA. Eur J Cancer 2000; 36: 1312–5
- Chan JM, Stampfer MJ, Giovannucci EL. What causes prostate cancer. A brief summary of the epidemiology. Semin Cancer Biol 1998; 8: 263–73
- Giovannucci EL, Clinto SK. Tomatoes, lycopene, and prostate cancer. Proc Soc Exp Biol Med 1998; 218: 129–39
- Giovannucci EL. Selenium and risk of prostate cancer. Lancet 1998; 352: 755–6
- Clark LC, Combs GF, Turnbull BW, Slate EH, Chalker DK, Chow J, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA 1996; 276: 1957–63
- Clark LC, Dalkin B, Krongrad A, Combs GF, Turnbull BW, Slate EH, et al. Decreased incidence of prostate cancer with selenium supplementation: Results of a double-blind cancer prevention trial. Br J Urol 1998; 81: 730–4
- Lowe JF, Frazee LA. Update on prostate cancer chemoprevention. Pharmacotherapy 2006; 26: 353–9
- Klein EA. Selenium and vitamin E cancer prevention trial. Ann NY Acad Sci 2004; 1031: 234–41
- Lippman SM, Goodman PJ, Klein EA, Parnes HL, Thompson JM, Kristal AR, et al. Designing the selenium and vitamin E cancer prevention trial (SELECT). J Natl Cancer Inst 2005; 97: 94–102
- Gianduzzo TR, Holmes EG, Tinggi U, Shahin M, Mactaggart P, Nicol D. Prostatic and peripheral blood selenium levels after oral supplementation. J Urol 2003; 170: 870–3
- Sabichi AL, Lee JJ, Taylor RJ, Thompson IM, Miles BJ, Tangen CM, et al. Selenium accumulation in prostate tissue during a randomized, controlled short-term trial of 1-selenomethionine: A Southwest Oncology Group Study. Clin Cancer Res 2006; 12: 2178–84
- Zachara BA, Szewczyk-Golec K, Wolski Z, Tyloch J, Skok Z, Bloch-Boguslawska E, et al. Selenium level in benign and cancerous prostate. Biol Trace Elem Res 2005; 103: 199–206
- Winnefeld K, Dawczynski H, Schirrmeister W, Adam G, Friedrich U, Hein S. Selenium in serum and whole blood in patients with surgical interventions. Biol Trace Elem Res 1995; 50: 149–55
- Thompson HJ, Wilson A, Lu J, Singh M, Jiang C, Upadhyaya P, et al. Comparison of the effects of an organic and inorganic form of selenium on a mammary carcinoma cell line. Carcinogenesis 1994; 15: 183–6
- Brooks JD, Weinstein M, Lin X, Sun Y, Pin SS, Bova GS, et al. CG island methylation changes near the GSTP1 gene in prostatic intraepithelial neoplasia. Cancer Epidemiol Biomarkers Prev 1998; 7: 531
- Ketterer B, Meyer DJ. Glutathione transferases: A possible role in the detoxication and repair of DNA and lipid hydroperoxides. Mutat Res 1989; 214: 33
- Lee W, Morton RA, Epstein JI, Brooks JD, Campbell PA, Bova GS, et al. Cytidine methylation of regulatory sequences near the pi-class glutathione-S-transferase gene accompanies human prostatic carcinogenesis. Proc Natl Acad Sci USA 1994; 91: 11733
- Ganther HE. Selenium metabolism, selenoproteins and mechanisms of cancer prevention: Complexities with thioredoxin reductase. Carcinogenisis 1999; 20: 1675
- Menter DG, Sabichi AL, Lippman SM. Selenium effects on prostate cell growth. Cancer Epidemiol Biomarkers Prev 2000; 9: 1171–82