Abstract
Background. Sentinel node biopsy (SNB) is a novel staging technique in cutaneous melanoma, but it is more challenging in the head and neck (H&N) than in the trunk and extremities. The aim of this study was to investigate the utility of SNB in patients with clinical stage I-II H&N cutaneous melanoma, with emphasis on disease outcome. Patients and methods. Twenty five patients with H&N melanoma of >1.0 mm in Breslow depth underwent SNB and were compared to 121 historic H&N melanoma patients, who had either undergone routine prophylactic neck dissection or had been observed without any invasive nodal staging. Results. Sixteen percent of the SNB patients were sentinel-positive and there have been no false-negative cases. In the Kaplan-Meier analysis, there were no significant differences between the study groups in melanoma-specific overall survival. Among the entire cohort, melanoma-specific overall survival rate was 67.1% at 5 years and 61.9% at 10 years. Predictive factors for worsen survival were nodal micrometastases, male sex, scalp location, thick primary lesion and ulceration. Discussion. SNB is a reliable and mini-invasive approach for the nodal staging of H&N cutaneous melanoma. Traditional neck dissection is recommended only for therapeutic purposes in clinically node-positive or sentinel-positive patients.
The sentinel node concept has altered the clinical practice of cutaneous melanoma and sentinel node biopsy (SNB) has widely replaced routine lymphadenectomy as the standard for surgical treatment. The landmark study on the subject was published by Morton et al. in 1992 Citation[1]. However, SNB has been reported to be more challenging in the head and neck (H&N) than in the trunk and extremities (T&E) Citation[2], Citation[25]. The sentinel nodes are often located near the primary lesion and the high background radioactivity may cause problems in their detection. The lymphatic drainage patterns of the skin are rich in the head and neck and more unpredictable than those in the other body sites. The harvesting of intraparotid sentinel nodes deserves special attention. These difficulties may stand behind a false-negative result in SNB leading to regional disease failure in tumour-bearing lymph nodes that have been left behind. On the other hand, there is no consensus whether the occult nodal micrometastases should be treated immediately or at an advanced stage; some authors are not willing to accept SNB as the standard of care Citation[3].
In Finland the incidence of melanoma has been steadily increasing. According to a nationwide database of the Finnish Cancer Registry Citation[4], the annual incidence rate of melanoma was 9.5 among male and 8.1 among female in 2001–2005 (age-adjusted rate per 100 000 population). During the same period the mortality rate was 2.2 in male and 1.0 in female, respectively. Within the Hospital District of Southwest Finland, the annual melanoma incidence has been slightly higher: 11.3 among male and 9.5 among female in 2001–2005. The catchment population is 460 000 residents within the hospital district. Approximately 10–15 patients with H&N melanoma are operated annually at the Department of Surgery in Turku University Hospital.
At our institution, a proportion of clinical stage I-II melanoma patients have undergone elective lymph node dissection (ELND) at the time of disease presentation during the past decades, but most patients have not undergone any invasive nodal staging until October 2001 when SNB was adopted for clinical use in the treatment of cutaneous melanoma. Since then, every SNB patient has been entered to a prospective database.
The aim of this study was to evaluate our first experience of the routine use of SNB and its feasibility in patients with clinical stage I-II H&N melanoma with Breslow depth of>1 mm. First, H&N melanoma patients who underwent SNB were compared to SNB patients with T&E melanoma, and secondly, to historical H&N melanoma patients who had either undergone routine ELND or who had not undergone any invasive nodal staging at the time of diagnosis and initial surgery.
Patients and methods
Prospective SNB group
From October 2001 to December 2006, a total of 159 consecutive patients with histopathologically confirmed invasive cutaneous melanoma, stage I-II (T2-4N0M0), Breslow depth over 1.0 mm, were enrolled onto a prospective database at the Department of Surgery, Plastic and Reconstructive Surgery Unit, Turku University Hospital, Turku, Finland. The study protocol was approved by the Institutional Review Board of the Turku University Hospital and each patient provided written informed consent. 25 of the 159 patients (16%) had a primary lesion in the head and neck region and 134 (84%) in the trunk or extremities.
All SNB patients underwent lymphatic mapping before the surgical procedure. The lymphoscintigraphy was performed by technetium-99m-labeled nanocolloid, which was injected intradermally at two to four points at the margins of the primary melanoma or the biopsy scar. After lymphoscintigraphy, SNB was performed within 20 hours. In the operation, a preoperative blue dye injection (Patent blue V) and intraoperative use of a gamma detecting probe (Navigator GPS, Tyco Health Care, Norwalk, CT) were used. All blue-stained and radioactive (ex vivo count exceeded 10% of the count of the most radioactive node) nodes were excised.
If no metatastatic melanoma cells were identified in the histopathological analysis in the hematoxylin and eosin-stained sections, further sections were cut and immunohistochemical staining with Melan-A and/or S-100 antibodies was performed. The patients who had micrometastases in their sentinel nodes underwent completion lymph node dissection, i.e. selective, modified radical or radical neck dissection.
After surgery, all patients were referred for further follow-up to the Department of Oncology and Radiotherapy, Turku University Hospital. No routine adjuvant therapy was used. The regular follow-up schedule consisted of initial staging by whole-body computed tomography and clinical examination every 3 to 6 months during the first 5 years.
Retrospective ELND and observation groups
For comparision, a retrospective cohort was collected from the time before SNB became routine, from January 1983 to September 2001. The data of 121 stage I–II cutaneous H&Nmelanoma patients were collected consecutively from the case records of Turku University Hospital. Ninety patients had been operated on at the Department of Surgery and 31 patients at the Department of Otorhinolaryngology–Head and Neck Surgery. Twenty nine of the patients had undergone elective lymph node dissection at the time of initial surgery (ELND group) and 92 had not undergone any invasive nodal staging (observation group).
The patients had been followed up at the Department of Oncology and Radiotherapy of Turku University Hospital or in the Satakunta Central Hospital, Pori, Finland. The follow-up schedule was similar to that described in the SNB group. The cause of death was obtained from autopsy reports and from Statistics Finland's Archive of Death Certificates.
Statistical analyses
Categorical variables were analyzed by the χ2 test and continuous data by Mann-Whitney nonparametric U test. Survival curves were constructed by the Kaplan-Meier method, and group differences were analyzed by the log rank test. The ticks along the curves in the survival plots represent cencored observations, including deaths from other causes than melanoma or unknown outcome. Univariate and multivariate survival analyses were performed by the Cox proportional hazard regression model; 95% confidence intervals (95% CI) were calculated. The starting point for all survival analyses was the time of initial melanoma treatment. A p-value less than 0.05 was considered statistically significant. Statistical analyses were performed with SPSS version 14.0 (SPSS, Chigaco, IL) software.
Results
A total of 146 H&N melanoma patients were evaluated: 69 male (47%) and 77 female (53%). The median age of the patients was 72 years (mean 67 years, range 10–92). The median Breslow depth was 3.0 mm (mean 3.6 mm, range 1.1–20.0). The primary lesion was located in the face in 83 patients (57%), in the scalp in 20 patients (14%), in the ear in 21 patients (14%) and in the neck in 22 patients (15%). The wound was closed directly in 55 patients (38%), by the use of a local flap in 70 patients (48%), and by skin grafting in 21 patients (14%).
SNB patients
In 22 H&N patients a total of 48 sentinel nodes were excised (mean 2.2 nodes per patient). Four patients were sentinel-positive (16%) and 18 patients were sentinel-negative (72%). Lymphatic mapping was unsuccessful and no sentinel nodes were indentified in three patients (12%). The distribution of the 48 sentinel nodes between regional nodal basins according to the location of the primary lesion is presented in . In six patients, one or more sentinel nodes were excised from the parotid gland and a micrometastasis was found in one patient. This patient underwent a superficial parotidectomy and a selective neck dissection of levels II–III in the second stage operation (). No complications of SNB were reported in the 22 patients.
Figure 1. 1A. A lymphoscintigram with four sentinel nodes of an 11-year-old girl with a melanoma in the middle of the left cheek. The primary lesion (Breslow depth 3.8 mm, Clark level IV) had originated in a benign Spitz nevus. 1B. A blue-stained sentinel node (II sn) found in the parotid gland. At the same time of SNB, the biopsy scar of the primary lesion was excised with 1.5 cm lateral margins and the wound was closed directly. 1C. Histopathological analysis (hematoxylin and eosin staining) revealed a subcapsular micrometastasis with minimal tumour burden. It was the only metastatic sentinel node. In a second stage operation, superficial parotidectomy and selective neck dissection of levels II–III were performed and no additional metastatic nodes were detected. 1D. Aesthetic result two years after the operation. The patient has remained disease-free after a follow-up of 27 months.
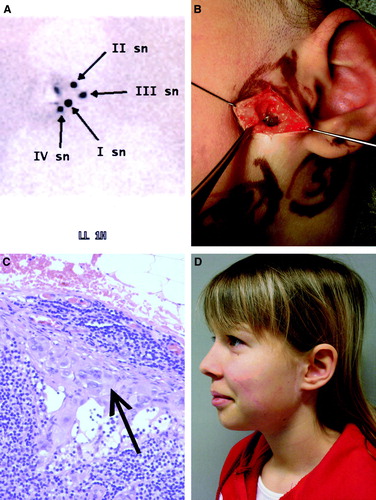
Table I. Distribution of sentinel nodes according to the location of the primary lesion
Comparison between study groups and nodal involvement
The clinical and histopathological charasterictics between SNB patients and control patients are presented in . At the time of disease presentation, nodal micrometastases were detected in four patients in both SNB and ELND groups (16% in the SNB group vs. 14% in the ELND group). During the follow-up, there has been one nodal recurrence in the SNB group; that patient was initially sentinel-positive and additional non-sentinel metastases were also detected in the subsequent neck dissection. There have been no false negative results of SNB. In the ELND group, there were six nodal recurrences, of which two patients had been node-positive and four patients node-negative, i.e. false-negative, at the time of initial surgery. In the entire cohort, nodal involvement, including both initial micrometastases and clinically detected late recurrences, was detected in a total of 37 nodal basins. The distribution of those metastatic nodal basins according to the location of the primary lesion is presented in .
Table II. Comparison between clinical and histopathological characteristics of SNB patients and control patients
Table III. Distribution of metastatic lymph node basins according to the location of primary lesion
Outcome
The median follow-up time was 27 months in the H&N melanoma SNB group (range, 8–65 months), 38 months in the ELND group (range, 6–218 months) and 46 months in the observation group (range, 1–260 months). In the SNB group there have been 7 recurrences (28% of the H&N melanoma SNB patients): 3 local, 1 nodal and 3 distal recurrences, determined as the location of first recurrence. In the ELND group there have been 11 recurrences (38% of the ELND patients): 1 local, 6 nodal and 4 distal. In the observation group there have been 35 recurrences (38% of the observation group patients): 3 local, 11 nodal and 12 distal. The number of melanoma-related deaths has been 3 in the SNB group (12%), 9 in the ELND group (31%) and 33 in the observation group (36%). Melanoma-unrelated deaths were categorized as censored observations.
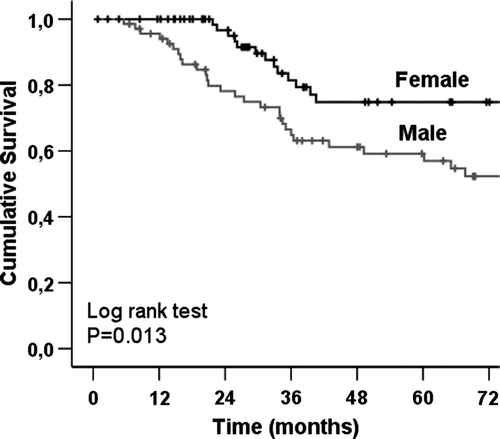
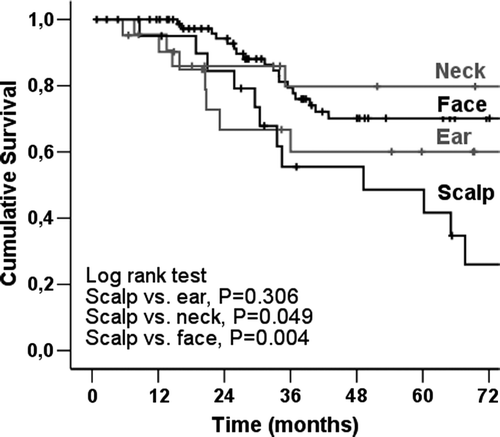
The melanoma-specific overall survival (OS) was 67.1% at 5 years and 61.9% at 10 years for the entire H&N melanoma group. In the Kaplan-Meier analysis, of 159 SNB patients there was no significant difference in melanoma-specific OS between H&N melanoma patients and T&E melanoma patients. There were no significant differences between the study groups (H&N SNB vs. ELND vs. observation) either (). In contrast, there was a significant difference between male and female in all of 146 H&N melanoma patients; the male were associated with a significantly poorer prognosis than the female (). The Kaplan-Meier curves by anatomic subsites indicate that patients with scalp melanomas have lower melanoma-specific OS rates compared with patients with face, neck and ear melanomas ().
Figure 2. Melanoma-specific overall survival of 25 SNB patients, 29 ELND patients and 92 observational patients.
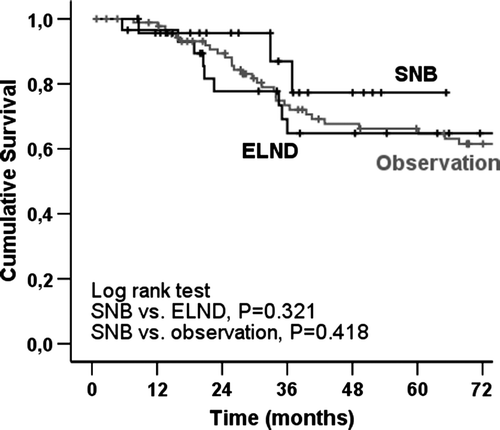
Figure 4. Melanoma-specific overall survival of 146 H&N melanoma patients according to the anatomic site of the primary lesion.
In the univariate analysis Breslow depth (hazard ratio, 1.41; 95% CI, 1.06–1.23; p=0.001), Clark level V (hazard ratio, 6.36; 95% CI, 1.37–29.5; p=0.018) and ulceration (hazard ratio, 2.96; 95% CI, 1.62–5.41; p<0.001) of the primary lesion were also predictive for poor survival. In the multivariate analysis only male sex was an independent prognostic factor (hazard ratio, 2.96; 95% CI, 1.18–7.38; p=0.020).
Discussion
Head and neck melanomas constitute approximately 17% of all cutaneous melanomas Citation[5]. However, melanoma density per skin area is clearly higher in the head and neck than in other body parts Citation[6], Citation[7]. This is particularly true in melanomas of the face, suggesting a higher incidence in sun-exposed skin areas. In our study populations, H&N melanoma patients were older than T&E melanoma patients (mean age 67 vs. 63 years). This finding is consistent with previous reports Citation[8], Citation[9]. In contrast to some other authors Citation[5], Citation[10], we did not find any predominance of males in this study (male 47% vs. female 53%).
Head and neck location is generally thought of as a negative prognostic factor for survival. Our results do not support this assumption. We have earlier reported on 921 melanoma patients with no significant difference in melanoma-specific OS between anatomic sites of the primary lesion by univariate or multivariate analysis Citation[11]. In this study, with different inclusion criteria, no significant difference in OS between H&E and T&E melanomas was detected in Kaplan-Meier analysis. Thus, there is no evidence of true location-related biologic aggressiveness of H&N melanomas. H&N melanomas may carry a statistically worse outcome because the primary lesions tend to be thicker and more advanced at the time of diagnosis than T&E melanomas, and in addition, the patients are older.
In the current study, a significant difference in melanoma-related OS was detected between the sexes (). We argue that the poor survival is not associated with the male gender itself. According to our results H&N melanomas of males were more often associated with scalp location (male:female ratio 4:1), thicker primary lesions (median Breslow depth, male 3.0 mm vs. female 2.8 mm) and a higher ulceration rate (male 42% vs. female 27%).
Our purpose was to investigate the feasibility of SNB as nodal staging compared with the traditional approach with either ELND or pure observation without any invasive nodal staging. The head and neck region is associated with complex and unpredictable drainage patterns. Multiple drainage basins have been reported in 36–44% of cases Citation[12], Citation[13]. Likewise, lymphoscintigrams have been discordant with clinical predictions in 34–43% of cases Citation[14], Citation[15]. In this study, we noticed that the first draining lymph nodes, i.e. sentinel nodes, were found in almost all neck levels and in the parotid gland (). The distribution of metastatic lymph node basins, when historical controls were included, was also similarly widespread (). The most common site for both sentinel nodes and metastases was neck level II found in approximately half of the cases.
Because of the multiplicity of lymphatic drainage of the head and neck, SNB is a technically demanding procedure compared with SNB in the groin or axilla. Indeed, lymphatic mapping in the head and neck is unsuccessful more often than in other body areas Citation[2]. In this study no sentinel nodes were visualized in the lymphoscintigraphy in 3 of 25 patients (12%). In the H&N region, sentinel nodes can be masked by high background radioactivity from the tracer injection site. In addition, the lymph nodes are often small and located in surgically demanding sites that are not easily accessible, as in the parotid gland. In this study 6 of 48 sentinel nodes were harvested from the parotid gland and one of them contained micrometastasis. In the entire cohort, 8 of 37 metastatic nodal basins (22%) were intraparotid.
SNB in the parotid gland may present special problems. Carlson et al. reported only a 79% success rate for parotid SNB Citation[13]. Intraparotid SNB is also associated with potential risk for complications i.e. facial nerve injury. Eicher et al. pointed out that after lymphatic mapping superficial parotidectomy or selective neck dissection including sentinel nodal basins should be performed routinely instead of SNB Citation[16]. Such an approach, however, compromises the mini-invasiveness of SNB. The subject has also been debated by Loree et al., Picon et al. and Ollila et al. Citation[17–19]. In contrast, their results suggest that intraparotid SNB is reliable, accurate and safe. Nevertheless, if the parotid gland contains sentinel nodes, their dissection should be performed meticulously by an experienced surgeon. The use of a nerve stimulator is recommended.
In this study, only 16% of H&N melanoma patients were sentinel-positive compared with 31% in other body regions, when Breslow depth was over 1.0 mm. Our result is in accordance with several other studies. Recently, Mattsson et al. reported a sentinel-positivity rate of 17% in patients with H&N melanoma versus 21% and 23% in truncal and leg melanomas, respectively Citation[20]. Leong et al. have published their results of the multi-institutional SLN Working Group on 629 H&N melanoma patients who underwent SNB. Only 10.1% were sentinel-positive, but thin T1-melanomas were also included Citation[21]. Chao et al evaluated 321 H&N melanoma patients and found a sentinel-positivity rate of 15%, which was significantly less than the 23% and 20% seen in truncal and extremity melanomas, respectively Citation[22]. It is not entirely clear why the incidence of nodal metastases in the head and neck is lower than expected. In some cases, the disease progression if present is associated rather with direct systemic dissemination than with step-wise lymphatic spreading after negative SNB. Furthermore, younger age is known to be a predictor of sentinel-positivity Citation[23]. Because H&N patients tend to be older, their low sentinel-positivity rate could be partly associated with their impaired lymphatic function. In this study, there were no false-negative cases suggesting that the sentinel node status accurately reflects the status of the relevant lymph node basin. However, only long-term follow-up will confirm the true sensitivity of SNB procedure.
Due to this reliability, in the era of SNB, it is doubtful whether there is any indication for routine ELND in clinical stage I–II melanoma. In our historical controls ELND detected nodal micrometastases in 14% of cases. Thus, the detection rate of micrometastases is rather comparable with that of SNB, but ELND is more invasive and is associated with a higher morbidity. Approximately 80% of patients are node-negative and they would be overtreated by ELND. After SNB, whole body positron emission tomography may provide additional information for staging in patients with high risk melanoma Citation[24], Citation[25].
The question if any nodal staging is superior to a pure observational approach is unclear from a therapeutic point of view. The survival benefit of SNB or ELND, if any, is very difficult to assess. Unfortunately, no effective adjuvant treatment is available in metastatic melanoma unlike in breast cancer. We could not demonstrate any survival advantage in this study either; SNB did not alter disease outcome significantly (). Interestingly, Fisher and al. published an analysis on 1 444 retrospective H&N melanoma patients and found that late nodal recurrences, which were treated by therapeutic lymph node dissection, were paradoxally associated with better survival compared with micrometastases, which were removed by ELND at the initial stage Citation[26]. However, there is no widespread support to such vaccine-effect of metastatic cells. In contrast, the results of the prospective randomized Multicenter Selective Lymhadenectomy Trial (MSLT-I) demonstrated an improved survival in node-positive patients with who had undergone SNB and completion lymph node dissection, and whose primary lesion was of intermediate-thickness Citation[27]. This favourable result is promising but it is based on a population with melanomas of all body sites lacking stratified data on particular anatomical sites. Until definitive evidence of therapeutic benefit is available, the patients have to be informed properly and SNB cannot be offered to them as a life-saving procedure in general. We fully agree with the conclusion pointed out by Tanis et al. in their recent review: there is clearly a need for a randomized controlled trial to enable evidence-based management in this particular group of patients with H&N melanoma Citation[28].
Indeed, the most obvious advantage of SNB is the unique prognostic information for nodal staging. Sentinel-positive patients carry a high risk for recurrence and need intensive follow-up. In the current study eight patients were upstaged either by SNB or ELND at the time of disease presentation. In six of them (75%) recurrent disease was detected during follow-up. In the future, these high risk patients are priority targets for adjuvant therapies Citation[29]. Until an effective form of systemic therapy is found, early diagnosis and adequate surgery remains the treatment of choice.
In conclusion, our study sample is small, but nevertheless, our results suggest that SNB is an acceptable staging tool in cutaneous head and neck melanoma. The presence of occult nodal micrometastasis detected by SNB is the most important prognostic factor in clinical stage I–II melanoma. SNB allows for selective application of therapeutic neck dissection only to nodal-positive patients. Instead of routine ELND, the majority of clinically N0 neck patients can be treated by a mini-invasive staging procedure.
References
- Morton DL, Wen DR, Wong JH, Economou JS, Gagle LA, Storm FK, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg 1992; 127: 392–9
- Jansen L, Koops HS, Nieweg OE, Doting MH, Kapteijn BA, Balm AJ, et al. Sentinel node biopsy for melanoma in the head and neck region. Head Neck 2000; 22: 27–33
- Thomas JM. Caution with sentinel node biopsy in cutaneous melanoma. Br J Surg 2006; 93: 129–30
- URL: http://www.cancerregistry.fi/eng/statistics/.
- Fisher SR. Cutaneous malignant melanoma of the head and neck. Laryngoscope 1989; 99: 822–36
- Gillgren P, Mansson-Brahme E, Frisell J, Johansson H, Larsson O, Ringborg U. Epidemiological characteristics of cutaneous malignant melanoma of the head and neck–a population-based study. Acta Oncol 1999; 38: 1069–74
- Hoersch B, Leiter U, Garbe C. Is head and neck melanoma a distinct entity? A clinical registry-based comparative study in 5702 patients with melanoma. Br J Dermatol 2006; 155: 771–7
- Agnese DM, Maupin R, Tillman B, Pozderac RD, Magro C, Walker MJ. Head and neck melanoma in the sentinel lymph node era. Arch Otolaryngol Head Neck Surg 2007; 133: 1121–4
- Kilpatrick LA, Shen P, Stewart JH, Levine EA. Use of sentinel lymph node biopsy for melanoma of the head and neck Am Surg 2007;73:754–8; discussion 758–9.
- O'Brien CJ, Coates AS, Petersen-Schaefer K, Shannon K, Thompson JF, Milton GW, et al. Experience with 998 cutaneous melanomas of the head and neck over 30 years. Am J Surg 1991; 162: 310–4
- Koskivuo I, Talve L, Vihinen P, Maki M, Vahlberg T, Suominen E. Sentinel lymph node biopsy in cutaneous melanoma: A case-control study. Ann Surg Oncol 2007; 14: 3566–74
- Wells KE, Rapaport DP, Cruse CW, Payne W, Albertini J, Berman C, et al. Sentinel lymph node biopsy in melanoma of the head and neck. Plast Reconstr Surg 1997; 100: 591–4
- Carlson GW, Murray DR, Lyles RH, Hestley A, Cohen C. Sentinel lymph node biopsy in the management of cutaneous head and neck melanoma. Plast Reconstr Surg 2005; 115: 721–8
- O'Brien CJ, Uren RF, Thompson JF, Howman-Giles RB, Petersen-Schaefer K, Shaw HM, et al. Prediction of potential metastatic sites in cutaneous head and neck melanoma using lymphoscintigraphy. Am J Surg 1995; 170: 461–6
- Lin D, Franc BL, Kashani-Sabet M, Singer MI. Lymphatic drainage patterns of head and neck cutaneous melanoma observed on lymphoscintigraphy and sentinel lymph node biopsy. Head Neck 2006; 28: 249–55
- Eicher SA, Clayman GL, Myers JN, Gillenwater AM. A prospective study of intraoperative lymphatic mapping for head and neck cutaneous melanoma. Arch Otolaryngol Head Neck Surg 2002; 128: 241–6
- Loree TR, Tomljanovich PI, Cheney RT, Hicks WL, Jr, Rigual NR. Intraparotid sentinel lymph node biopsy for head and neck melanoma. Laryngoscope 2006; 116: 1461–4
- Picon AI, Coit DG, Shaha AR, Brady MS, Boyle JO, Singh BB, et al Sentinel lymph node biopsy for cutaneous head and neck melanoma: Mapping the parotid gland Ann Surg Oncol 2006. DOI: 10.1245/ASO.2006.03.051
- Ollila DW, Foshag LJ, Essner R, Stern SL, Morton DL. Parotid region lymphatic mapping and sentinel lymphadenectomy for cutaneous melanoma. Ann Surg Oncol 1999; 6: 150–4
- Mattsson J, Bergkvist L, Abdiu A, Aili Low JF, Naredi P, Ullberg K. et al Sentinel node biopsy in malignant melanoma: Swedish experiences 1997–2005 Acta Oncol (in press).
- Leong SP, Accortt NA, Essner R, Ross M, Gershenwald JE, Pockaj B, et al. Impact of sentinel node status and other risk factors on the clinical outcome of head and neck melanoma patients. Arch Otolaryngol Head Neck Surg 2006; 132: 370–3
- Chao C, Wong SL, Edwards MJ, Ross MI, Reintgen DS, Stadelmann WK, et al. Sentinel lymph node biopsy for head and neck melanomas. Ann Surg Oncol 2003; 10: 21–6
- Sondak VK, Taylor JM, Sabel MS, Wang Y, Lowe L, Grover AC, et al. Mitotic rate and younger age are predictors of sentinel lymph node positivity: lessons learned from the generation of a probabilistic model. Ann Surg Oncol 2004; 11: 247–58
- Koskivuo IO, Seppänen MP, Suominen EA, Minn HR. Whole body positron emission tomography in follow-up of high risk melanoma. Acta Oncol 2007; 46: 685–90
- Tai CJ, Hsu CH, Chiou JF, Wu CH, Lin SE. FDG uptake in a rectal malignant melanoma. Acta Oncol 2007; 46: 1030–31
- Fisher SR. Elective, therapeutic, and delayed lymph node dissection for malignant melanoma of the head and neck: Analysis of 1444 patients from 1970 to 1998. Laryngoscope 2002; 112: 99–110
- Morton DL, Thompson JF, Cochran AJ, Mozzillo N, Elashoff R, Essner R, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med 2006; 355: 1307–17
- Tanis PJ, Nieweg OE, van den Brekel MW, Balm AJ. Dilemma of clinically node-negative head and neck melanoma: Outcome of “watch and wait” policy, elective lymph node dissection, and sentinel node biopsy–A systematic review. Head Neck 2008; 30: 380–9
- Stadler R, Luger T, Bieber T, Köhler U, Linse R, Technau K, et al. Long-term survival benefit after adjuvant treatment of cutaneous melanoma with dacarbazine and low dose natural interferon alpha: A controlled, randomised multicentre trial. Acta Oncol 2006; 45: 389–99