Encouraging results have been seen in a 5-year follow-up of patients diagnosed with glioblastoma and treated with temozolomide during and after postoperative radiotherapy. Patient survival was almost 10% in the combination group compared to less than 2% in the radiotherapy alone group Citation[1]. We have also seen clinical reports Citation[2], Citation[3], further substantiated in the present evaluation by Skovgaard-Poulsen and colleagues Citation[4], that an antiangiogenic approach could be of significant clinical value in recurrent high-grade glioma. The positive outcome from using an antiangiogenic approach is well in line with one of the biologic hallmarks of glioblastoma, i.e. an excessive and abnormal vessel formation. Vascularization is also a WHO histopathological criterion to determine the grade of a brain tumour Citation[5]. Moreover, a negative correlation between extent of vascular density and favourable prognosis of the disease is evident and the VEGF/VEGFR signaling pathways affecting endothelial cells is known to be of tremendous importance for the neovascularization in malignant glioma Citation[6], Citation[7]. Moreover, other approaches using tumour vasculature as the target in brain tumours are also under development Citation[8–10].
The study by Skovgaard-Poulsen in the present issue of Acta Oncologica Citation[4] is a retrospective evaluation of previous heavily pretreated patients with recurrent malignant brain tumours. The patients received the anti-VEGF antibody bevacizumab (10 mg/kg) and the topoisomerase-1-inhibitor irinotecan (the dose was adjusted to if enzyme-inducing antiepileptica was used or not) every 2 weeks in a schedule similar to the one used in colorectal cancer. Most of the tumours were high grade glioma (23 patients with glioblastoma and 13 with Grade 3 anaplastic astrocytoma of 47 evaluable patients). As can be seen from the results, the combination of bevacizumab and irinotecan is associated with clinically significant and durable objective responses, including clinical improvement and measurable “complete responses” using MRI/PET. The study also gives clear indication that these responses were transformed into prolongation of life, not at least when emphasizing the dismal prognosis in this patient population. Although the number of patients treated is limited, the efficacy seems to be more pronounced in patients with glioblastoma compared to the other patients treated. This might reflect that glioblastoma is usually associated with an intense neovascularisation and VEGF expression and, thus, may be more sensitive and a more attractive target for anti VEGF treatment with drugs like bevacizumab. The significance of this assumption that VEGF expression could be a marker for response of an antiangiogenic drug is of interest to further evaluate since we today have no clinically relevant biomarker in other tumours treated with bevacizumab. The angiogenic profile has also just recently been proposed by others to predict radiographic response and survival in malignant astrocytoma Citation[11], Citation[12].
The results of the Danish study and the other reports are definitively hopeful and in line with our own experiences with significant clinical responses, considerable symptom relief and unexpected long survival in various heavily pretreated glioma patients (exemplified in ). Interestingly, even if most of the results so far obtained are focused on glioblastoma, this antiangiogenic approach seem to be of value to evaluate also in other brain tumours and according to our limited experience not restricted to the use of irinotecan, but even seen in combination with PCV and temozolomide. In fact, a combination of radiotherapy, temozolomide and bevacizumab in up-front treatment of 10 patients with newly diagnosed glioblastoma has been shown to be feasible with more than 8 months PFS Citation[12].
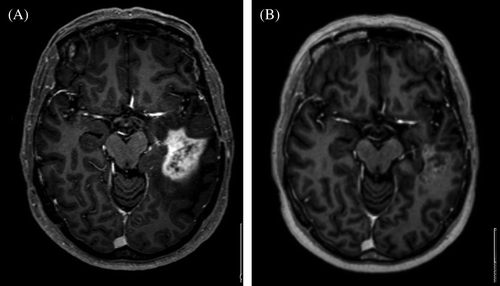
Figure 1. (T1 weighted gadolinium enhanced MRI scans ). A 54 year old female patient diagnosed with glioblastoma September 2006. She was primary treated with postsurgical radiotherapy- temozolomide and adjuvant temozolomide. One year later the disease progressed. Re-operation was not possible and chemotherapy with PCV schedule was started. Due to further progress valganciklovir was initited. In january 2008 after confirmed progressive disease (A) treatment was initiated with bevacizumab and irinotecan according to the schedule described in the paper. Four months later she was in a good condition without any corticosteroids, working as a hairdresser (B).
Another facet to the discussed clonogenic tumour cell death, reduction in tumour cell metabolic activity and the subsequent reduction in tumour burden following bevacizumab, is the challenging effects caused by “normalization” of tumour blood vessels and seen as a reduction in vascular permeability Citation[9], Citation[13]. Thus, by targeting VEGF the capillary leakage may be reduced and brain-oedema relieved. Anti-VEGF treatment may therefore be a long awaited option to corticosteroids in combating brain-oedema and elevated intracranial pressure. This kind of effect has also been seen using VEGFR-tyrosine kinase inhibitors, such as cediranib Citation[9], Citation[10].
Even if the data is compelling when combining bevacizumab and irinotecan a lot of remarks have already been raised and some issues both from basic scientific and clinical settings remains to be elucidated. The activity of irinotecan as a single agent in malignant glioma has been shown to be limited with no obvious survival benefit, though it has a good penetration to the CNS. The rationale for using this regimen is obviously based on experience in colorectal cancer. Furthermore, this combination was also challenged at the last ASCO meeting when it was shown that only bevacizumab was as effective as the combination schedule Citation[14]. The use of other cytotoxic drugs with a more encouraging single activity must also be evaluated in combination with the antiangiogenic approach before this regimen is established in the routine setting of recurrent glioma.
Another important issue to deal with is to balance the encountered positive effects with increased toxicities. Combining chemotherapy with anti-VEGF approaches may be expected to increase fatigue, myelotoxicity and the risk for trombo-embolic events. Noteworthy, the number of patients known from publications that have been treated with bevacizumab based regimens is still just around 100. Therefore rare, but clinically important, adverse effects could have been missed. In fact, craniotomy site wound dehiscence has been reported to be a unique cumbersome adverse effect following antiangiogenic treatments in brain tumour patients, and problems with local wound healing, at least in close proximity (weeks) to surgery, following bevacizumab treatment have been observed Citation[12].
Thus, there is today an accumulation of challenging data which demonstrate that we hopefully are in a new era in the management of patients with highly malignant brain tumours, not at least suggested by the antiangiogenic approach seen in the study by Skovgaard-Poulsen and colleagues Citation[4]. This concept could in the final end be a brilliant example of how you in the profession, outside the routine management, develop and early adopt a valuable therapy for a patient group with poor outcome. Nevertheless, saying that, the limited number of patients so far evaluated, a risk of potentially harmful effects in the long run and requirement of increased resources, emphasize the need of further well-balanced and controlled studies. These studies must also include aspects of translational science, evaluating potential predictive marker in order to guide future treatment decision-making. Even the greatest enthusiast must accept a thorough scientific and clinical evaluation before any kind of treatment can be fully adopted in the clinical setting. But, for how long will patients suffering from recurrent glioblastoma accept to wait?
References
- Miramanoff M. Unpublished data presented at Perspectives in CNS Malignancies IV in Berlin March 28-29, 2008
- Stark-Vance V. Bevacizumab and CPT-11 in the treatment of relapsed malignant glioma. J Neurooncol 2005;7:369( Abstract 342).
- Vredenburgh JJ, Desjardins A, Herndon JE, Marcello J, Reardon DA, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol 2007; 25: 4722–9
- Skovgaard-Poulsen H, Grunnet K, Sorensen M, Hasselbach B, Nelausen K, Kosteljanetz M, et al Adult primary brain tumour patients with a history of multiple recurrences: Results of treatment with an angiogenesis inhibitor, bevacizumab, combined with irinotecan. Acta Oncol ( in press).
- Louis DN, Oghaki H, Wiestler OD, Cavenee WK. WHO classification of tumours in the central nervous system. 2007; IARC.
- Hicklin DJ, Ellis LM. Role of the VEGFR pathway in tumour growth and angiogenesis. J Clin Oncol 2005; 23: 1011–27
- Johansson M, Brännström T, Bergenheim AT, Henriksson R. Spatial expression of VEGF-A in human glioma. J Neurooncol 2002; 59: 1–6
- Sandström M, Johansson M, Bergström P, Bergenheim AT, Henriksson R. Effects of the VEGFR inhibitor ZD6474 in combination with radiotherapy and temozolomide in an orthotopic glioma model. J Neurooncol 2008; 88: 1–9
- Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT. Angiogenesis in brain tumours. Nat Rev Neurosci 2007; 8: 610–22
- Batchelor T, Sorensen G, di Tomaso E, Zhang W-T, Guda D, et al. AZD2171, a pan VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 2007; 11: 83–95
- Sathornsumetee, Cao Y, Marcello JE, Herndon II JE, McLendon RE, Desjardins A. Tumor angiogenic and hypoxic profiles predict radiographic response and survival in malignant astrocytoma patients treated with bevacizumab and irinotecan. J Clin Oncol 2008;26:271–8.
- Lai A, Filka E, McGibbon B, Nghiemphu PI, Graham C, Yong W, et al. Phase II pilot study of bevacizumab in combination with temozolomide and radiotherapy for up-front treatment of patients with newly diagnosed glioblastoma multiforme: Interimanalysis of safety and tolerability. Int J Radiat Oncol Biol Phys 2008; 71: 1372–80
- Ananthnarayan S, Bahng J, Roring J, Nghiemphu P, Lai A, et al Time course of imaging changes of GBM during extended bevacizumab treatment. J Neurooncol 2008.
- Cloughesy TF, Prados MD, Wen PY, Mikkelsen T, Abrey LE, Schiff D, et al A phase II, randomized, non-comparative clinical trial of the effect of bevacizumab (BV) alone or in combination with irinotecan (CPT) on 6-month progression free survival (PFS6) in recurrent, treatment-refractory glioblastoma (GBM). ASCO 2008, Chigaco. Abstract 2010b.