To the Editor
In May 2000, a 23-year-old male underwent thymectomy and adjuvant radiotherapy for Masaoka stage II thymoma with positive resection margins. In December 2003, CT and positron emission tomography (PET) showed biopsy-proven bilateral pleural recurrence. Left-sided surgical pleurodesis was followed by four cycles of CAP chemotherapy (cisplatin, doxorubicin, cyclophosphamide) with complete remission on CT and PET. However, bilateral pleural disease recurred in March 2005, and he received four cycles of ChlVPP chemotherapy (chlorambucil, vinblastine, procarbazine and prednisolone) and radiotherapy to the left chest wall and diaphragm. Residual disease was seen on CT and PET and he pursued non-conventional therapies over the next 12 months.
Upon return in September 2006, he had multiple new pulmonary nodules and progressive pleural disease. In June 2007 there was significant pulmonary and pleural progression, and he was enrolled onto a phase IB study of motesanib diphosphate (AMG 706) in advanced solid tumours. Motesanib diphosphate is a novel oral, highly specific inhibitor of vascular endothelial growth factor receptor-1 (VEGFR-1), VEGFR-2, VEGFR-3, Kit and platelet-derived growth factor receptor (PDGFR).
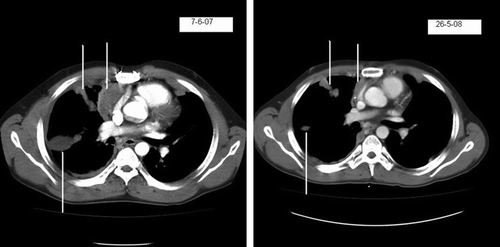
The patient received 75 mg of motesanib diphosphate twice daily in three week cycles (two weeks on treatment, one week off). He had stable disease by RECIST (Response Evaluation Criteria in Solid Tumours), although there was a modest increase in tumour measurements that reached a maximum after 11 cycles (January 2008). Subsequently there was improvement, and after 17 cycles (May 2008) there had been a 28% decrease in tumour measurements from maximum (see ). He remains on study as of July 2008, having received motesanib diphosphate for over 12 months.
Figure 1. CT scans done at baseline (7/6/07) and after 17 cycles (26-5-08) of treatment with motesanib diphosphate showing reduction in pleurally-based masses.
Vascular endothelial growth factor-A (VEGF-A) and VEGFR-1 & 2 are uniformly expressed in both normal and malignant thymic tissue Citation[1]. Despite this, there is a paucity of data regarding anti-angiogenesis agents in advanced thymic epithelial tumours. Combined treatment with bevacizumab 15 mg/kg q3w and erlotinib 150 mg/day was evaluated in a phase II trial of 18 patients with recurrent thymic epithelial tumours Citation[2]. No patients achieved a response, but 11 of 18 (60%) had stable disease. In a phase 1 study of escalating doses of aflibercept, a soluble decoy receptor that binds VEGF-A, and docetaxel 75 mg/m2, one patient with thymoma achieved a partial response Citation[3]. Two patients with thymoma also had a partial response in a phase I trial of SU014813, an oral inhibitor of VEGFR2, PDGFR, Kit, and Fms-related tyrosine kinase 3 (Flt-3) Citation[4].
In a previous phase 1, dose-finding study of motesanib diphosphate in 71 patients with advanced solid tumours, five patients (7%) achieved a partial response and 35 (49%) stable disease Citation[5]. Three of the five responders had advanced thyroid cancer. This led to a phase II study of motesanib diphosphate in 93 patients with advanced thyroid cancer Citation[6], in which the response rate was 14%, and stable disease was achieved in 67% of patients. The clinical trial that our patient is enrolled in is still in progress and results are not currently available.
One patient with advanced thymoma was enrolled in the aforementioned phase 1 study of motesanib diphosphate. This patient was a 49-year-old female with chemotherapy-refractory thymoma. She also attained clinical benefit from motesanib diphosphate, having stable disease for eight months while on study.
We present a very rare case of motesanib diphosphate displaying clinical activity in advanced thymoma. In our patient with chemotherapy-refractory disease, motesanib diphosphate achieved a meaningful reduction in tumour measurements. Interestingly, the patient did not have evidence of actual tumour reduction until after 13 cycles (i.e. 39 weeks), illustrating that the benefit of treatment with targeted agents such as motesanib diphosphate can be delayed. While the response to treatment falls just short of a partial response by RECIST, we believe that further evaluation of anti-angiogenesis drugs such as motesanib diphosphate is warranted for chemotherapy-refractory advanced thymic epithelial tumours.
Acknowledgements
We would like to thank Amgen Inc. for supporting this work. We would also like to acknowledge the efforts of the Austin Hospital Cancer Clinical Trials Centre.
We wish to declare the following:
P. L. R. Mitchell was a member of the Amgen advisory panel in lung cancer in 2006;
N. C. Tebbutt and S. White are conducting research sponsored by Amgen.
References
- Cimpean AM, Raica M, Encica S, Cornea R, Bocan V. Immunohistochemical expression of vascular endothelial growth factor A (VEGF), and its receptors (VEGFR1, 2) in normal and pathologic conditions of the human thymus. Ann Anat 2008; 190: 238–45
- Bedano PM, Perkins S, Burns M, Kessler K, Nelson R, Schneider BP, et al. A phase II trial of erlotinib plus bevacizumab in patients with recurrent thymoma or thymic carcinoma. J Clin Oncol 2008; 26(Suppl), (Abstract 19087)
- Isambert N, Freyer G, Zanetta S, Falandry C, Soussan Lazard K, Fumoleau P. A phase I dose escalation and pharmacokinetic (PK) study of intravenous aflibercept (VEGF trap) plus docetaxel (D) in patients (pts) with advanced solid tumors: Preliminary results. J Clin Oncol 2008; 26(Suppl), (Abstract 3599)
- Fiedler, WM, Giaccone, G, Lasch, P, Van der Horst, I, Brega, NM, Raber, S, , et al. Phase I trial of SU014813 in patients (pts) with advanced solid malignancies. J Clin Oncol ( Meeting Abstracts). 2007;25(18 Suppl):3521.
- Rosen LS, Kurzrock R, Mulay M, Van Vugt A, Purdom M, Ng C, et al. Safety, pharmacokinetics, and efficacy of AMG 706, an oral multikinase inhibitor, in patients with advanced solid tumors. J Clin Oncol 2007; 25: 2369–76
- Sherman SI, Wirth LJ, Droz JP, Hofmann M, Bastholt L, Martins RG, et al. Motesanib diphosphate in progressive differentiated thyroid cancer. New Engl J Med 2008; 359: 31–42
