Medical physics is destined to be ever-changing. It has emerged as a discipline that is responding to technological and scientific problems faced in the medical field – either by developing and introducing new imaging and treatment technologies, or by discovering and implementing new and existing methodologies. Over the years, medical physics has had a profound impact on the medical practice, particularly in terms of improved diagnostics and treatment of the disease.
One could hardly imagine a field of physics that is more dynamic and has a brighter long-term future. This privilege does not come for free however; it requires medical physicists to have broad scientific interests, to constantly learn and acquire new knowledge, and always be ready for surprises that might change the direction of their work.
Because of this particular dynamics of medical physics, questions about its future seem to appear more often than in other fields. Even though predicting the future is not possible, general development of the field can be anticipated by reviewing the factors and forces that drive its development – developments in medicine and other related fields, unique expertise that medical physicists possess, and demonstrated flexibility of the field in the past. In the light of developments in the medical physics field, it should not be overseen that medical physics is not the only physical/engineering discipline on the intersection with medicine. Developments in medical physics sometimes strongly overlap with developments in related disciplines like biomedical engineering and biophysics.
This overview focuses on current trends that are already impacting medical physics field. It is by no means an exhaustive review of recent developments, but it is aimed to highlight some of the specific areas, which might have the most significant and profound impact on the long-term future of the field. In addition, it was aimed to provide some personal views what the main challenges the field faces, and what are some directions that medical physicists should embark on to secure a long-term high-impact position in the medical field.
Dramatic changes in medicine require changes in medical physics
Medicine is facing dramatic changes itself. While medicine has not been able to prevent and cure the diseases, it has managed to prevent and cure many lethal acute conditions, turning them into long-term chronic conditions. Given this dramatic shift from acute to chronic disease, which also represent a significant financial burden on the societies, the strategies for preventing and treating diseases are beginning to shift. Probably the most important long-term affect will be in identifying an individual's susceptibility to a disease, in prevention, early diagnosis, reduction of complications, and smarter therapies. This leads to what is sometimes termed “the 4 P's of Medicine”: medicine that will be more Predictive, Personalized, Preemptive, and Participatory.
What this entails can be best depicted from the recent letter to the U.S. Congress by Dr. Zerhouni (http://www.nih.gov/strategicvision.htm), the director of the U.S. National Institutes of Health, where he states: “To reach these key long-term goals, NIH is strategically investing in research to further our understanding of the fundamental causes of diseases at their earliest molecular stages. But individuals respond differently to environmental conditions, according to their genetic endowment and their own behavior. In the future, research will allow us to predict how, when, and in whom a disease will develop. We can envision a time when we will be able to precisely target treatment on a personalized basis to those who need it, avoiding treatment to those who do not. Ultimately, this individualized approach will allow us to preempt disease before it occurs, utilizing the participation of individuals, communities, and healthcare providers in a proactive fashion, as early as possible, and throughout the natural cycle of a disease process.”
This long-term future of medicine is also financially supported through multiple strategic initiatives, summarized in the NIH roadmap, launched in 2003 (http://nihroadmap.nih.gov/) Citation[1]. It consists of three main components termed: (1) New pathways to discovery, (2) Research teams of the future and (3) Re-engineering clinical enterprise. The “New pathways to discovery” is aimed at advancing understanding of the daunting complexity of biological systems. The complexity of the problems requires “Research teams of the future” where scientists would move beyond the confines of their own discipline and explore new organizational models for team science. In order to make research clinically effective “Re-engineered clinical enterprise” should enable quick translation of basic research discoveries into drugs, treatments, or methods for prevention.
The question arises where medical physics fits into this picture. Are we even willing and ready to change? What are the main practical challenges we are facing?
Medical physics has always been closely connected with technology development. A lot of research and development that we today think of as medical physics actually happened in general physics and was then applied to medicine and biology Citation[2]. However, technology development is becoming also more and more demanding, complex and expensive, practically limiting what can be done in an academic research environment. Because of this, it is to expect that most of the technology development will be done in corporate environment rather than in academia. Forming and maintaining strong collaborations and partnership with the companies is thus essential.
Approach to disease diagnostics is also becoming more and more complex. Diagnostic procedures are becoming extensive, utilizing a variety of diagnostic tools, from imaging procedures to various biomarker testing, like genetic and molecular profiling. In addition, more and more therapies are being combinatory, combining traditional modes of therapy like radiation therapy, with molecular targeted therapies. For medical physicists, interdisciplinary knowledge will be essential to be an appropriate discussion and collaborative partner in this process.
Because of the complexity of diagnostic and treatment procedures, the team disease management has become much more a common. In addition to physician teams (e.g., radiation oncology + medical oncology + surgery teams), disease management often includes other interdisciplinary scientists, like pathologists, pharmacologists, molecular biologists. While medical physicists traditionally do interact with a certain type of physicians, most notably radiation oncologists and radiologists, interaction with other basic scientists is much less common.
Fast advances in life sciences represent new research challenges in medicine. We have just started to peak into the complexity of biological systems. We are getting more and more data, from imaging and biomarker assessments. In addition, new technologies like nanotechnology and stem cell engineering are likely to introduce dramatic changes to how medicine will be practiced. While medical physicists are good at solving this type of problems, it seems we have not really seriously tapped into them.
In summary, medical physicists are good at developing and refining technology, which should remain our main focus. On the other hand, medical physicists are not good in life sciences, which is something that will have to be improved. Medical physicists are good at interacting with physicians and other basic scientists, but it is essential to become much better at it. Finally, medical physicists have knowledge and skills to attack the problems of modern medicine, but should not be shy at embarking on new territories.
Two areas of particular importance to keep the future of medical physics bright as it is now are new research initiatives and education and training that would lead to new generation of medical physicists. Both of the topics will be discussed with some particular examples.
New research directions
While it is impossible to predict which areas of research are most likely to gain attention of medical physicists, it is possible to speculate, based on previous discussion, which of the directions might be of the highest interest to the medical field. It should not be neglected that most of medical physicists are currently involved in radiation therapy of cancer. Consequently, future developments in radiation oncology will have the strongest impact on the majority of medical physicists, at least in the near future. On the other hand, the diversity of medical physics as well as the strong connection to other similar disciplines, like biomedical engineering might bring other areas into the focus or my cause that some of the areas will find home in other physical and engineering disciplines. Some of the major areas, which will be briefly discussed here, are:
Personalization of therapy
Molecular imaging
Chemotherapy and molecular targeted therapies
Particle therapies
Clinical trials
Translational research
Simulations of complex systems
Another important question is where to obtain patient and tumour specific biological information that will allow dose painting. There is a general consensus that the most realistic way to identify biological heterogeneity of the tumours in vivo, or to characterize response to therapy is to use functional and molecular imaging techniques Citation[9], Citation[10]. However, several obstacles need to be overcome before this goal is achieved. Currently, all molecular imaging technologies provide only better or worse surrogates of the underlying biological processes. Sometimes advanced kinetic analysis modelling is required to extract the biological information of interest from dynamic imaging datasets. Furthermore, certain molecular processes can be observed with a variety of different imaging modalities. For example, in order to assess tumour perfusion, one has an option to choose between CT perfusion, MR perfusion, or PET perfusion imaging, or, in order to assess tumour hypoxia, one has to choose between a variety of PET imaging agents, or BOLD MRI or less direct measures. What are the advantages, disadvantages, limitations and associated uncertainties with each of these modalities? How do these modalities perform for different anatomical and tumour sites? Why would one pick one over the other? Another prominent problem in biomedical imaging is the quantification of the imaging data. Since most of biomedical imaging has been developed for diagnostic purposes where the primary goal is tumour detection, exact quantification was always secondary. However, when the aim is to characterize and quantify biological properties of the tumours or quantify treatment response, the problem of having reliable numbers in the imaging data becomes critical. These are very complex problems, which require in-depth understanding of the performance of different modalities, as well as good knowledge of different options that the modalities offer. Again, medical physicists are uniquely positioned to address these problems.
Use of molecular imaging to design personalized therapy is opening doors for medical physicists to be involved in research, traditionally not included in the medical physics research spectrum, like chemotherapy and molecular targeted therapies. Many questions need to be answered, for example what type of imaging should be used to assess therapy, how to best extract useful information from imaging, how to make imaging most quantitative, how to analyse and interpret imaging data, how to optimally use imaging resources, how to connect imaging most effectively with other biomarkers etc. Again, medical physicists should be important, if not the key players to answer these questions.
While particle therapies are not new to medical physics, the recent increase in interest will likely have a profound impact on the future of radiation oncology. More effective sparing of normal tissue given the same dose to the tumour provides potential for both, more effective tumour control as well as less toxic therapies Citation[11], Citation[12]. While the cost of these systems are currently preventing their wide-spread use, the challenge of making the systems more effective, more flexible and cheaper represents a welcome challenge for many medical physicists, as well as engineers. Use of the new technologies is also linked with questions that have been answered in photon and electron therapy, but need to be fully addressed in particle therapies like accurate dosimetry and treatment planning, treatment verification, image guidance. Heavy involvement of medical physicists in development of new and more efficient particle systems as well as optimization of their use is guaranteed.
Medical physicists should become compulsory members on all clinical trials that involve advanced imaging and therapy, regardless whether the clinical trial is focused on radiation therapy, chemotherapy or molecular targeted therapies. Incorporation of imaging into clinical procedures also involves consideration of clinical protocol requirements and endpoints, good knowledge of patient anatomy, physiology, and most importantly tumour biology. If, in addition to radiation therapy, molecular targeted therapies are used, understanding of mechanisms of action and pharmacodynamics is essential. Similarly, molecular imaging is often used in conjunction with a variety of molecular markers; therefore, a basic understanding of their function, predictive power, and accuracy is important. Clearly, in this case, medical physicists can only be members of interdisciplinary teams. In order to be constructive and pertinent members of the teams, it is essential for medical physicists to have enough knowledge about basics of cell and molecular biology, particularly related to tumour biology, as well as anatomy, physiology and radiology. It is unrealistic to expect that medical physicists would be experts in these fields, but they should know enough to be adequate partners.
Translational research is another area, where traditionally medical physicists have not been significantly involved. Interestingly, while medical physicists are extremely active in clinical research, involvement in basic research, with the exception of radiobiology, is not very common. Part of the reason might be the lack of their basic biological knowledge; however, also their typical employment within clinical radiation oncology departments might contribute to this situation. Developing the tools for basic research, helping with the design and analysis of small animal experiments, connecting the results to clinical studies are the worthwhile tasks for medical physicists. Particularly involvement in imaging and radiation therapy aspects of preclinical studies, where medical physicists have most experience from clinical work, is of utmost importance.
Physicists have typically a great ability of abstraction, designing and testing models and performing model simulations. Medical physicists have mastered modelling and simulations of particle transport, as well as modelling of the whole treatment planning chain. Expansion of their knowledge and expertise to simulations of complex biological systems should not be difficult from a technical perspective. In addition, access to the biological information, either through biological imaging or other biomarkers, as well as access to the environment and resources where additional preclinical experiments can be performed make medical physicists ideal scientist to lead the efforts. Of course the biological complexity of the systems, most typically tumours, exceeds the knowledge that medical physicists typically have. Therefore, a strong collaboration with cell and molecular biologists, as well as physicians is essential to approach such problems.
The discussed possible research topics are only a subset of available research directions for medical physics. However, even this short overview indicates that the unique expertise that medical physicists have provides solid foundation to expand the current research horizon of medical physics.
Training and education
If we summarize the main differences between research orientation of medical physicists at present and in the future, we can see that currently research is:
predominantly done with little cross-disciplinary interaction,
with some interaction with physicians, most often radiation oncologists and radiologists,
typically has a significant clinical relevance.
In the future, medical physics research will have to be:
much more inter- and trans-disciplinary,
with much more interaction with physicians as well as other basic scientists,
will have to have do, in addition to clinically relevant, also biologically relevant research.
An example of the University of Wisconsin (UW) IRAT is presented with a particular emphasis on involvement of medical physicists (). The UW IRAT has four components: Initial Protocol Assessment Team (IPAT), Imaging Protocol Advisory and Review Teams (IPARTs), Clinical Imaging Shared Facility (CISF) and Image Analysis Core (IMAC).
Figure 1. University of Wisconsin Image Response Assessment Team (IRAT) structure. Note several key components – IPAT, IPARTs, CISF and IMAC. This structure enables to follow protocols from the initial protocol design through protocol implementation and protocol result analysis. Note involvement of medical physicists on multiple levels.
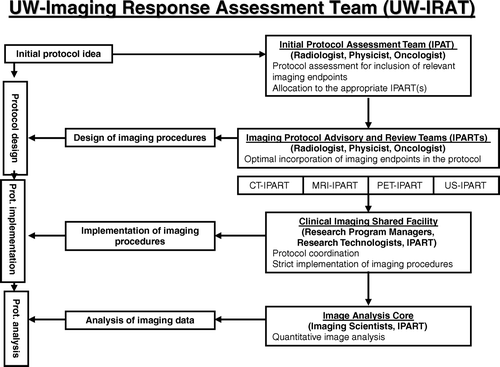
In the initial planning phase, every clinical protocol is reviewed by the Initial Protocol Assessment Team (IPAT), consisting of a panel of radiologists, medical physicists and oncologists. Together with the primary investigator, the IPAT determines appropriateness and the type of imaging procedure(s) that could be incorporated into a particular protocol. Imaging procedures are determined according to the desired scientific endpoints as well as local expertise for given imaging procedures. Based on this initial evaluation, the IPAT determines appropriate Imaging Protocol Advisory and Review Team (IPART), to which the protocol is referred. The IPART teams consist of specialized radiologists and physicists familiar with the intricacies of image acquisition techniques that are needed for specific oncologic applications. IPARTs are involved in protocol design and execution and meet with the particular clinical trial Principal Investigator, with whom they review, advice and help with the imaging aspects of protocol development. Clinical Imaging Shared Facility (CISF) is a shared resource which coordinates the review, development, and implementation of all clinical research protocols that contain an imaging component. Particular emphasis is made to increase QA/QC of imaging procedures, required in sequential imaging necessary in the treatment response context. Image Analysis Core (IMAC) is another essential component of the clinical trial workflow that is responsible for quantitative analysis of imaging data. Imaging data have to be analyzed accurately, reproducibly and timely. Image analyses can range from simple analysis (e.g., image normalization, ROI delineation) to more comprehensive analysis (e.g., compartmental kinetic modelling), as is often required for extraction of functional and molecular information from dynamic imaging datasets. In cases where multiple imaging modalities are involved or where multiple imaging is performed the analysis becomes even more laborious and challenging as they require proper normalization and co-registration of patient anatomies. Typically, the same IPART team, involved in the protocol design and implementation, oversees image analysis as well. This ensures self-consistency of the study.
The IRAT mechanism has proven to be a very effective way that stimulates incorporation of imaging, and it also ensures that imaging is included into the protocol in the most optimal way. Most importantly, the key players in the whole IRAT mechanism are also medical physicists. While for most medical physicists such activity is typically beyond their regular activities, it should be seen as an exciting opportunity to expand the domain of medical physics.
This is a good example to raise a question whether medical physicists are properly trained to be involved in such interdisciplinary teams. If we look at the American Association of Medical Physicists (AAPM) membership structure () we observe the following discrepancy (http://www.aapm.org).
Figure 2. Structure of physicists within AAPM according to their main work area. Distribution among therapy, diagnostic and both areas physicists is shown on the left and distribution between primarily diagnostic and therapy by age is shown on the right.
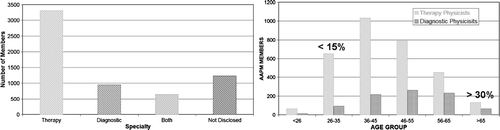
One can observe that a large majority of medical physicists are involved in therapy. According to a recent survey more than 50% of AAPM members declared to be predominantly therapy physicists. If the undecided physicists are distributed according to the decided physicists, almost 70% of physicists see themselves as predominantly therapy physicists. On the other hand, only 20% of AAPM members see themselves as predominantly imaging physicists. If we look at the historical distribution, we see that while more than 30% of physicists were imaging physicists in the past, this ratio has significantly decreased to less than 15% of young medical physicists who see themselves as predominantly imaging physicists. Increased emphasis of imaging will definitely change this ratio. Even more likely is that the classical separation of physicists between therapy and imaging will start to disappear and in the future most of the physicists will have to be skilled in both therapy and imaging.
One of the key components of the transition from present to future is the response of professional societies, like AAPM, ESTRO, EFOMP, and IOMP to the new challenges. If the societies respond favourably and in an organized fashion, so will the broader membership. The societies should help with the directions, guidelines and recommendations that help educate, guide and increase the awareness of the current research challenges among the membership. Several years ago, it was recognized within AAPM that the traditionally separate therapy and imaging sections should merge. For this reason, a specific Joint Imaging/Therapy section has been formed within the AAPM structure, first to respond to the growing interests of therapy physicists in imaging applications, and second to promote imaging among the traditional therapy-only physicists. In addition, strong emphasis has been put on increasing awareness of, and stimulating interest in more image-related research in the traditional therapy research setting, primarily through the increased number of continuing education sessions and symposia targeting the intersection between imaging and therapy. Just glancing through the 2008 AAPM annual meeting program, one can see that of 21 conference symposia 15 symposia are imaging related (8 imaging and 7 joint: imaging/therapy) and only 6 are therapy related. Interestingly, even among the only 6 therapy related symposia, two have a strong radiobiological flavour, leaving only 4 symposia, or less than 20% of the total, for therapy topics. If we compare this to the AAPM membership base (Figure 3), where over 70% of the members work predominantly in the therapy setting, the discrepancy between the current reality and future direction of medical physics is more than apparent. The response is happening also on the teaching and education committee, where the recent AAPM TG. 79 report on “Academic program recommendations for graduate degrees in medical physics” from 2002 is about to be revised, and updated to reflect new educational needs for medical physicists.
The response of professional societies is only the first step. The key to the success of preparing medical physicists for the future is basic training and education. One can also not miss out the strongest driving force – regulations, sometimes created by medical physicists themselves, sometimes by the regulatory bodies. For example, in the USA the recent requirement from American College of Radiology (ACR), the main clinical medical physics accreditation body, that a prerequisite for certification in radiologic physics after 2012 would be completion of a CAMPEP accredited program created a lot of concern how to meet this goal giving the shortage of such programs in the USA. In addition, inequality in training requirements between medical doctors and medical physicists have led to initiatives to establish a professional degree, so-called doctorate of medical physics (DMP), which would be awarded after master degree and appropriate clinical residency.
Similarly in Europe, the requirements of the 97/43/EURATOM (1997) directive, which defines Medical Physics Experts as a required professional in medical procedures involving radiation has triggered more organized medical physics education structure within EFOMP members. Together with the requirements of the Bologna process, where most of the graduate academic training is restructured, that provides good ground for many European medical physics programs to perhaps re-think how to prepare most efficient and into-the-future oriented programs. Rather than base programs on the traditional medical physics curricula, special care should be taken to incorporate enough imaging, biological and translational research components into the programs. Rather than preparing medical physicists for the present, the programs should prepare them for the future. One of the problems is that all dedicated medical physics programs, as well as other educational programs from where people enter the field of medical physics, have typically a very limited biological training. The knowledge of biology is most often limited to ‘high school’ training. In spite of the fact that medical physics has always interacted with life sciences, there has never been such a clear need to understand the basics of the cellular and molecular mechanisms governing disease development and treatment. With the emerging molecular imaging and other molecular diagnostic techniques, it is time to start thinking of a more systematic training in this area. There is no need for in-depth training, but the medical physicist should at least learn the basic language, learn about potential, but also limitations and problems in these cross-disciplinary fields. Just as basic knowledge of anatomy and physiology has become a required standard for medical physicists, so should the basic knowledge of cell and molecular biology.
Conclusion
Medical physics is facing significant changes, particularly with quick development of biological sciences, more complex research requiring interdisciplinary teams and strong need for translational research. The changes towards personalized medicine are opening new avenues for medical physicists like molecular imaging and extending beyond radiation therapy. In order to prepare medical physicists for the future, education and training should be properly adjusted including more basic non-physical sciences, particularly biology, more imaging, especially molecular imaging, and with more interdisciplinary and translational research components. Only in this way will we secure long-term future for medical physics.
References
- Zerhouni E. Medicine. The NIH Roadmap. Science 2003; 302(5642)63–72
- Webb S. The contribution, history, impact and future of physics in medicine. Acta Oncol 2008.
- Brahme A, Ågren AK. Optimal dose distribution for eradication of heterogeneous tumours. Acta Oncol 1987; 26: 377–85
- Levin-Plotnik D, Hamilton RJ. Optimization of tumour control probability for heterogeneous tumours in fractionated radiotherapy treatment protocols. Phys Med Biol 2004; 49: 407–24
- Webb S. Optimum parameters in a model for tumour control probability including interpatient heterogeneity. Phys Med Biol 1994; 39: 1895–914
- Webb S, Nahum AE. A model for calculating tumour control probability in radiotherapy including the effects of inhomogeneous distributions of dose and clonogenic cell density. Phys Med Biol 1993; 38: 653–66
- Ling CC, Humm J, Larson S, Amols H, Fuks Z, Leibel S, Koutcher JA. Towards multidimensional radiotherapy (MD-CRT): Biological imaging and biological conformality. Int J Radiat Oncol Biol Phys 2000; 47: 551–60
- Bentzen SM. Theragnostic imaging for radiation oncology: Dose-painting by numbers. Lancet Oncol 2005; 6: 112–7
- Gambhir SS. Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer 2002; 2: 683–93
- Weissleder R. Molecular imaging: Exploring the next frontier. Radiology 1999; 212: 609–14
- Schulz-Ertner D, Tsujii H. Particle radiation therapy using proton and heavier ion beams. J Clin Oncol 2007; 25: 953–64
- Jakel O, Schulz-Ertner D, Karger CP, Nikoghosyan A, Debus J. Heavy ion therapy: Status and perspectives. Technol Cancer Res Treat 2003; 2: 377–87