Abstract
Background. Estrogen receptor-α (ERα) is an important prognostic and predictive marker in breast cancer. ERα signaling normally down-regulates expression of Apolipoprotein D (ApoD), a lipocalin that binds, transports or chelates lipophilic ligands, including tamoxifen (TAM). Hence, the co-expression of ApoD may therefore identify clinical relevant subgroups of ERα positive breast cancer patients. Material and methods. ApoD, ERα, and progesterone receptor (PR) protein expressions were determined by immunohistochemistry (IHC) in primary tumors of 290 patients with operable breast cancer. The median follow-up was 12 years. Patients were stratified according to age, nodal stage and the expression of ERα and the combined cytoplasm and nuclear staining of ApoD (ApoDCN). Results. In elderly women (≥70 years) (n = 76), ApoDCN expression identified different prognostic subgroups in ERα positive patients (Trend: p < 0.0001). Multivariate analysis in this age group (n = 72), showed that the ERα-positive /ApoDCN-negative subgroup had a better breast cancer specific survival (BCSS) compared with the ERα-positive/ApoDCN-positive group (hazard ratio (HR) = 4.3; 95% CI = 1.6–11.9; p = 0.005). This difference was predominantly seen in the node positive patients (n = 30) (HR = 10.5; 95% CI = 2.3–47.6; p = 0.002). In a subset of postmenopausal ERα-positive/node positive patients (n = 60) previously enrolled in a trial on 2 year adjuvant TAM 20 mg vs. placebo, a better BCSS was observed in ApoDCN negative patients compared to placebo (p = 0.02). In ApoDCN positive patients, adjuvant TAM did not provide any survival benefit. Discussion. ERα and ApoDCN co-expression seems to be of prognostic importance in node positive elderly patients with operable breast cancer. In addition, we hypothesize that ApoDCN expression may be a novel marker and/or mechanism of TAM resistance in postmenopausal node positive patients. Thus, when targeting the ERα pathway in these patients, the ApoD status of the tumor may be of clinical relevance.
Estrogen receptor-alpha (ERα) has been regarded as an important initiator and promoter of tumor development Citation[1], and a useful prognostic factor for relapse and disease survival. Determination of ERα status has been of importance for prognostication, and for prediction of tailored anti-estrogen treatment. This treatment option is of relevance in elderly patients in particular, due to a higher risk of detrimental side effects when systemic cytotoxic adjuvant treatment is employed Citation[2]. ERα positive tumors are more commonly encountered in postmenopausal and elderly patients Citation[3]. However, clinical effects of anti-estrogen treatment do not always parallel these higher ERα expression rates Citation[4]. Likely, there might be various phenotypically subgroups of ERα positive tumors with different clinical courses.
The lipocalin Apolipoprotein D (ApoD = Gross Cystic Disease Fluid Protein-24 (GCDFP-24) = Progesterone Binding Cyst Protein (PBCP) Citation[5]) thought to be a simple transport molecule for small lipophilic ligands including progesterone and arachidonic acid Citation[6], has recently been associated with cytoprotection by binding or chelating toxic substances Citation[7]. Cell line studies have shown that ApoD expression is strongly repressed by the ERα action Citation[8]. Moreover, tamoxifen (TAM) up-regulates ApoD levels Citation[9], probably through an indirect mechanism, by inhibition of the ERα signaling. TAM binding to ApoD Citation[10] may indicate a triangular relationship between ApoD, ERα, and TAM Citation[6].
In a recent paper, we report an adverse outcome in operable breast cancer patients ≥70 years with a combined expression of ApoD in the cytoplasma and nuclei (ApoDCN) of the cancer cells Citation[11]. We have previously suggested that effects of adjuvant TAM are related to tumor cytosol content of PBCP (=ApoD) in node positive patients with operable breast Citation[12]. In the present study, we evaluate if ApoDCN expression in the invasive front of the primary tumor may be of importance for survival, and may predict effects of adjuvant tamoxifen in elderly and postmenopausal patients with ERα-positive tumors and operable breast cancer.
Material and methods
Patients
An overview of the patients included in this study is outlined in . Between 1983 and 1987, 443 consecutive women with operable breast cancer (Stage I and II) from Western Norway were included in a study on the possible prognostic relevance of cytosol PBCP content in primary breast cancers, as reported previously Citation[12]. From the original study population, 331 patients had confirmed invasive breast cancer (i.e.>2 mm diameter) and available tissue blocks for the recent study Citation[11], and also for the present study. Among 112 (25%) excluded patients, 20 had ductal carcinoma in situ (DCIS I-III) or micro-invasive cancer < 2 mm, four had poor quality of the histological material and the remaining 88 patients had not available tissue blocks for immunohistochemical (IHC) analysis. There were no statistically significant or clinically important differences between the original (n = 443) and the present study group (n = 331) with regard to age, tumor size, nodal status, clinical stage, menopausal status and progesterone receptor content (data not shown). Due to technical reasons, information on ERα and ApoDCN expression was available in 290 of these 331 patients. Sixty node positive postmenopausal patients from our study population were enrolled in a previous conducted randomized trial of adjuvant TAM 20 mg daily vs. placebo for 2 years Citation[13]. The distribution of basic patients characteristics (e.g. pT status) in the tamoxifen and control arm for both the ApoDCN positive and ApoDCN negative patients was similar to the distribution in the original study population Citation[13]. These patients were analyzed separately for possible predictive effects of ApoD expression. Postmenopausal status was defined as the clinical status of menopause. Patients ≥70 years of age were defined as elderly.
Figure 1. Flowsheet over patients included in the present paper. The 1st sub-analysis was done to evaluate a possible role of ApoDCN co-expression with ERα in the elderly patients ≥ 70 years. The 2nd sub-analysis evaluated the potential influence of ApoDCN status on the predictive effect of tamoxifen in the ERα positive postmenopausal patients with node positive disease included in the NBCG-1 trial [13]. DCIS, ductal carcinoma in situ; y, years; ERα, estrogen receptor alfa; ApoDCN, Combined cytoplasmic and nuclear ApoD staining; pN+, node positive; NBCG-1, Norwegian Breast Cancer Group adjuvant study # 1 [13].
![Figure 1. Flowsheet over patients included in the present paper. The 1st sub-analysis was done to evaluate a possible role of ApoDCN co-expression with ERα in the elderly patients ≥ 70 years. The 2nd sub-analysis evaluated the potential influence of ApoDCN status on the predictive effect of tamoxifen in the ERα positive postmenopausal patients with node positive disease included in the NBCG-1 trial [13]. DCIS, ductal carcinoma in situ; y, years; ERα, estrogen receptor alfa; ApoDCN, Combined cytoplasmic and nuclear ApoD staining; pN+, node positive; NBCG-1, Norwegian Breast Cancer Group adjuvant study # 1 [13].](/cms/asset/0617a0a1-7487-47c5-8df5-2f945b485dbf/ionc_a_362229_f0001_b.gif)
Tumor specimens
The tumor size (pT-stage) was measured in the fresh surgical breast tumor specimens and all detectable axillary lymph nodes were prepared for histology as described previously Citation[11]. The median number of identified lymph nodes was 9 (range, 1–36). Revision of the histological classification and grading of the tumors was done by experienced pathologists (EG, JPAB) according to WHO criteria Citation[14]. Tumor category (pT) and nodal status (pN) were reclassified according to the UICC criteria from 2002 Citation[15], which did not alter the distribution of the patients between the pT1/pT2 and pN0/pN+ subgroups compared with the original classification of the patients Citation[12].
Immunohistochemistry
ApoD, ERα and progesterone receptor (PR) were determined by immunohistochemistry (IHC) in 1.7 mm tissue microarray (TMA) cylinders carefully selected from the invasive front of the tumor. The invasive front of the tumor is important for the evaluation of various biomarkers in malignant tumors Citation[16], and is recently shown to be relevant for prognostication related to ApoD expression Citation[11]. The invasive front had to fulfill the following criteria Citation[16]: a) most cellular area, b) in the periphery of the tumor and c) inflamed or necrotic areas or areas in contact with the epidermis should be avoided.
The antigen retrieval was performed by using a highly standardized retrieval system (ImmunoPrep, Instrumec, Oslo, Norway) and the immunohistochemical technique was based on DAKO Cytomation technology (DAKO, Glostrup, Denmark) described in details recently Citation[11]. In short, immunostaining was performed on 4 µm sections adjacent to the H&E section used for diagnosis by using an autostainer (DAKO). The sections were incubated with the antibodies (ApoD, clone 36C6, Novocastra, 1:200 dilution; ER-α, clone SP-1, NeoMarkers, 1:400 dilution and PR, clone SP-2, NeoMarkers, 1:400 dilution) for 30 min.
The quantitation of ApoD in immuno sections has been described in detail elsewhere Citation[17]. In brief, the amount of ApoD expressed in the cytoplasm and nuclei were semi-quantitatively assessed by the H-score method Citation[11], Citation[17]. For ERα and PR, the percentage of positively stained nuclei was calculated by dividing the number of all positive staining intensities by the total number of counted cells.
Due to 13–14% loss of tumor tissue during the TMA production process, ApoD H-score and ERα percentage could be calculated in 290 (87%) patient samples, while all three immuno biomarkers (ApoD, ERα and PR) were available in 284 (86%) patients.
Cut-off levels
A count of ≥10% positive cells is commonly used as a threshold for ERα and PR positivity, and was also applied in the present study. As shown recently Citation[11], a H-score of 0 vs.>0 was found by receiver operating characteristic curve (ROC) analysis to be the optimal threshold value for both for cytoplasmic and the ApoD nuclear staining in postmenopausal and elderly women.
ERα and ApoD subgroups
The combined cytoplasmic and nuclear localization (ApoDCN) has recently been demonstrated to be of highest prognostic relevance compared to other staining patterns Citation[11]. We therefore made four subgroups according to the ApoDCN and ERα status. Except from a lower median age (53 years) in the single ERα − /ApoDCN− sub-group, various characteristics of the ERα/ApoDCN subgroups (n = 290) were similar ().
Table I. Comparison of features between subgroups of ERα /ApoDCN status (n = 290).
Endpoints
Relapse free survival (RFS) was defined as the time from the primary operation until confirmation of a relapse (i.e. recurrent disease of any location, or a new malignant tumor in the contra lateral breast). Breast cancer specific survival (BCSS) was defined as the time from the primary operation until death from breast cancer. Cause of death was provided from hospital records and in few cases also by information from the patient's general physician.
Statistics
SPSS v. 13.0 for Macintosh (SPSS, Chicago, IL, USA) and MedCalc (MedCalc Statistical Software v. 9.3.7, Mariakerke, Belgium) were used for statistical calculations. Category variables were analyzed with the ?2 test, and Fisher's exact test was applied when appropriate. Survival estimates were calculated using the Kaplan-Meier method, and univariate comparisons of survival curves were performed with the log-rank test. Stratification of age groups was done according to treatment guidelines from the Norwegian Breast Cancer Group (NBCG) Citation[18]; and survivals were calculated and compared for these age groups. Multivariate analysis was performed with the Cox proportional hazards method using backward and forward stepwise models with the following variables: tumor size category (pT1/pT2), nodal status (pN0/pN+), histological grade (WHO grade 1–3), PR positive/negative, and the four different ERα/ApoDCN expression subgroups as shown in . The “Log-minus-log” plot confirmed a constant hazard rate ratio during the observation period.
In the elderly patients, the subgroup (ERα − /ApoDCN−) comprised only four patients , and these were therefore excluded from the survival analysis. Two-tailed p-values < 0.05 were considered statistically significant.
Ethics
The Regional Ethics Committee (#151.04), the Norwegian Social Science Data Service (#11241), the Norwegian Institute of Public Health, Bio Bank Registry (#1500), and the Norwegian Data Inspectorate (#2004/1432-2) approved all aspects of this study.
Results
ApoDCN is inversely correlated to ERα in postmenopausal and elderly patients
An inverse correlation between ERα and ApoDCN expression was observed in patients ≥70 years (r = − 0.43; p < 0.0001). This was also true for all postmenopausal patients (r = − 0.13; p = 0.045). No correlation was found between ERα and ApoDCN in pre-menopausal patients. There was no correlation between PR and ApoDCN for any age groups. A positive correlation (r = 0.35; p< 0.0001) was found between ERα and PR for all age groups. Due to the correlation between ERα and ApoDCN in the elderly, interaction analysis between these two variables was done, and no interaction was found in the multivariate analysis (data not shown). Accordingly, a possible independent influence of ApoDCN categories on ERα subgroups was further evaluated.
Survivals
The ERα/ApoDCN subgroups showed prognostic relevance in patients ≥70 years, both for RFS and BCSS both in the univariate () and in the multivariate analysis (). Of note is a significant different RFS of the subgroups with ERα positive tumors, related to the ApoDCN co-expression (p = 0.004, log-rank) (Figure 2a). In addition, the ERα + /ApoDCN+ and the ERα − /ApoDCN+ subgroups had an almost similar RFS (A). Regarding BCSS, a more favorable outcome was seen in elderly patients with ERα + /ApoDCN− tumors, as compared to the ERα + /ApoDCN+ group (p = 0.004, log-rank) (B).
Figure 2. A. Relapse free survival (RFS; all locations) of the elderly patients ≥ 70 years. Grouping is based on ApoDCN and ERα expression in the cytoplasm. A significant difference in RFS (p = 0.004) was found between group 1 (ERα + /ApoDCN−) and group 2 (ERα + /ApoDCN+). Of note is the observation of no significant difference (p=0.39) in RFS between group 2 (ERα + /ApoDCN+) and group 3 (ERα − /ApoDCN+). We excluded the ERα − /ApoDCN− tumors from the analysis in this age group, due to a low number of patients (n = 4).B. Breast cancer specific survival (BCSS; dead of breast cancer) in the elderly patients > 70 years. Stratification is based on ERα/ApoDCN category of the primary tumor. A statistically significant survival difference (p = 0.004) between group 1 (ERα + /ApoDCN−) and group 2 (ERα + /ApoDCN+) was observed, and also between group 2 and group 3 (ERα − /ApoDCN+) (p = 0.03). We excluded the ERα − /ApoDCN− tumors from the analysis in this age group, due to a low number of patients (n = 4).
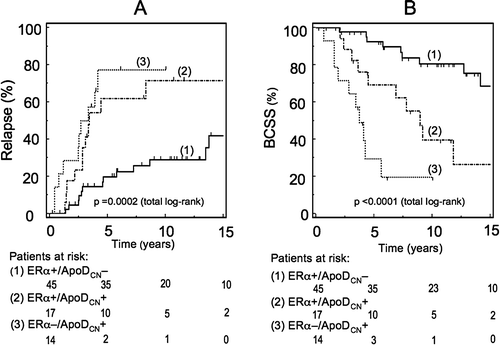
Table II. Univariate survival analysis of patients ≥70 years of age (n = 76)
Table III. Multivariate analysis of patients ≥70 years of age (n = 72)
This prognostic association between BCSS and ERα/ApoDCN subgroups in elderly patients was only seen in the node positives (p = 0.003). In addition, the ERα + /ApoDCN+ combination showed a HR of 10.5 (95% CI = 2.3–47.6) when compared to the reference group. No statistically significant survival differences associated to ERα/ApoDCN subgroups were seen in the node negative elderly patients (data not shown).
ApoDCN status – a possible predictor of adjuvant effect of TAM in postmenopausal node positive patients
At the time of diagnosis and primary treatment 60 postmenopausal patients comprised in our total study population (), were included in a prospective randomized controlled clinical trial on adjuvant TAM 20 mg daily vs. placebo for 2 years Citation[13]. In our current evaluation, we have used the IHC-ERα information to categorize the ER status (10% cut-off). Patients with ERα + /ApoDCN+ tumors who received adjuvant TAM had a BCSS at 15 years similar to patients receiving placebo (A). In contrast, the estimated BCSS was improved by 30% in patients with ERα + /ApoDCN− tumors who received adjuvant TAM (p = 0.02) (B).
Figure 3. A. Breast cancer specific survival (BCSS) in the ApoDCN− tumors of postmenopausal patients enrolled in a prospective randomized adjuvant study (Adjuvant study NBCG − 1) on 20 mg tamoxifen (TAM) for 2 years vs. placebo (i.e. Control = Ctr) [13].B. Breast cancer specific survival (BCSS) in the ApoDCN+ tumors of postmenopausal patients enrolled in the prospective randomized adjuvant study (Adjuvant study NBCG – 1) on 20 mg tamoxifen (TAM) for 2 years vs. placebo (i.e. Control = Ctr) [13].
![Figure 3. A. Breast cancer specific survival (BCSS) in the ApoDCN− tumors of postmenopausal patients enrolled in a prospective randomized adjuvant study (Adjuvant study NBCG − 1) on 20 mg tamoxifen (TAM) for 2 years vs. placebo (i.e. Control = Ctr) [13].B. Breast cancer specific survival (BCSS) in the ApoDCN+ tumors of postmenopausal patients enrolled in the prospective randomized adjuvant study (Adjuvant study NBCG – 1) on 20 mg tamoxifen (TAM) for 2 years vs. placebo (i.e. Control = Ctr) [13].](/cms/asset/33cd51d7-8449-497f-9bc1-c3846d828699/ionc_a_362229_f0003_b.gif)
Discussion
Our present study, based on patients with operable breast cancers, shows an inverse relationship between ERα and ApoDCN expression in the postmenopausal and elderly patients (i.e. ≥70 years of age). This observation is partly in line with Citation[12], but is also contradictory to Citation[19] previous studies. Different study populations (i.e. 33% of the patients had T3 or T4 tumors) and methodological differences Citation[19] can partly explain the discrepancies. Intra-tumoral heterogeneity of ApoDCN expression Citation[17] may disturb correlation studies when using whole sections Citation[19]. We have used a consistent sampling approach by TMA from the invasive front in the periphery of the tumor. Accordingly, serial TMA sections may resemble the cell line studies Citation[8] more than whole sections do. Since ERα expression in breast cancer tissue increases with age Citation[3], correlations between ERα and ApoDCN may be easier to detect in the elderly.
Detrimental aspects of ApoDCN co-expression in ERα+ tumors in elderly patients (A & B) are of note. Previously, ApoD expression was regarded as a marker of a functional steroid-signaling pathway Citation[8], indicating a well-differentiated tumor with a favorable prognosis Citation[19]. However, the three strong transcriptional inhibitory ERα responsive elements in the promoter region of the ApoD gene Citation[20] support an inverse relationship between ERα and ApoD expression provided by a functional ERα signal pathway. Therefore, a co-expression of ApoDCN and ERα may reflect a dysfunctional ERα signaling. The present observation of a shorter RFS and BCSS in elderly patients, when ERα and ApoDCN are co-expressed, may reflect such a dysfunctional ERα pathway. The molecular basis for this dysfunction could be mutations in the ERα gene (ERS-1 gene) Citation[21] or changes in various co-factors required for functional ERα signaling. In line with this suggestion, a dysfunctional pathway may lead to a phenotypically “intermediate” ERα positive tumor with a more aggressive disease in comparison with tumors harboring a normal ERα signaling. As reported recently from an animal model, the ERα signaling was damaged in MCF-7 cells that had spread to regional lymph nodes Citation[22]. Hence, node positive breast cancers may be in a different stage of disease where ERα signaling is increasingly damaged. Effects of a dysfunctional ERα signaling may be more visible in elderly patients, rarely given chemotherapy Citation[2], Citation[18]. Likewise, cytotoxic adjuvant treatment more commonly offered to younger age groups may also conceal these differences. Whether or not a dysfunctional ERα signaling pathway occurs more frequently in elderly node positive patients need to be evaluated.
Once expressed in the tumor cell, ApoDCN may act independently as a prognostic determinant through its putative protective features of the cancer cell Citation[7], which may include binding and chelating of tamoxifen Citation[10]. As observed previously Citation[12], and in line with our present observations based on an extended follow-up time and improved determination methods, a possible predictive value of ApoDCN with regard to effects of adjuvant TAM treatment is hypothesized. Also, in line with our prognostic considerations of the ERα/ApoDCN co-expression, the predictive information of the ApoDCN may be linked to an altered function of the ERα pathway. This may be part of the complex scenario encountered in endocrine resistant breast cancers, consequently with reduced clinical effects of tamoxifen treatment Citation[23].
In spite of our well-defined study population, comprising women with operable breast cancer, treated according to national guidelines at that time, and the use of modern and reproducible determination methods for ERα and ApoDCN, interpretation of our observations should be done with caution. Available patients and number of events for evaluation and statistical calculations are reduced, but the previous Citation[12] and our present study population was comparable with regard to important clinical and tumor characteristics. The concern regarding lower numbers should be partially made up for by the long follow-up time, which significantly exceeds follow-up times commonly encountered in comparable reports Citation[19]. While this is of particular concern regarding our subgroups analysis Citation[13], we think our observations add biological information to be further evaluated in other studies.
If ApoD expression may indicate a less than well recognized de novo mechanism of TAM resistance in breast cancer, remains to be addressed in appropriate studies. The fact that TAM is converted into different identifiable active metabolites Citation[24] makes it necessary to focus on the possible affinity of ApoD to these metabolites in particular, before any conclusions can be made.
Our study indicates that co-expression of ApoDCN in ERα positive tumors may impair the prognosis in elderly women with node positive breast cancer. Improved understanding of the ERα and its signaling pathway in this particular age group is of importance. The role of ApoDCN expression in breast cancer is less than well elucidated and further studies to explore subcellular mechanisms and possible clinical effects are warranted.
Acknowledgements
Håvard Søiland, MD is a research fellow financially sponsored by the Western Norwegian Health Authorities (project # 911166). Financial support from The Norwegian Cancer Society, the L. Meltzer Foundation and O. Mjålands Foundation is highly appreciated. The authors are grateful for the technical assistance from Irene Øvestad and Marit Nordhus. We thank Associate Professor Jan Terje Kvaløy, PhD, Department of Mathematics and Natural Sciences, University of Stavanger for statistical guidance.
References
- Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med 2006; 354: 270–82
- Bernardi, D, Errante, D, Galligioni, E, Crivellari, D, Bianco, A, Salvagno, L, , et al. Treatment of breast cancer in older women. Acta Oncol Epub [cited September 26 2007] PubMed; PMID 17899452.
- Pierga JY, Girre V, Laurence V, Asselain B, Dieras V, Jouve M, et al. Characteristics and outcome of 1755 operable breast cancers in women over 70 years of age. Breast 2004; 13: 369–75
- Early Breast Cancer Trialists' Collaborative Group. Tamoxifen for early breast cancer: An overview of the randomised trials. Lancet 1998;351:1451–67.
- Balbin M, Freije JM, Fueyo A, Sanchez LM, Lopez-Otin C. Apolipoprotein D is the major protein component in cyst fluid from women with human breast gross cystic disease. Biochem J 1990; 271: 803–7
- Søiland H, Søreide K, Janssen EA, Kørner H, Baak JP, Søreide JA. Emerging concepts of Apolipoprotein D with possible implications for breast cancer. Cell Oncol 2007; 29: 195–209
- Do Carmo S, Levros LC, Jr, Rassart E. Modulation of Apolipoprotein D expression and translocation under specific stress conditions. Biochim Biophys Acta 2007; 1773: 954–69
- Simard J, Dauvois S, Haagensen DE, Levesque C, Merand Y, Labrie F. Regulation of progesterone-binding breast cyst protein GCDFP-24 secretion by estrogens and androgens in human breast cancer cells: A new marker of steroid action in breast cancer. Endocrinology 1990; 126: 3223–31
- Harding C, Osundeko O, Tetlow L, Faragher EB, Howell A, Bundred NJ. Hormonally-regulated proteins in breast secretions are markers of target organ sensitivity. Br J Cancer 2000; 82: 354–60
- Lea OA. Binding properties of progesterone-binding cyst protein, PBCP. Steroids 1988; 52: 337–38
- Søiland, H, Janssen, EAM, Kørner, H, Varhaug, JE, Skaland, I, Gudlaugson, E, , et al. Apolipoprotein D predicts adverse outcome in patients over 70 years with operable breast cancer. Breast Cancer Res Treat Epub [cited March 11 2008]. PubMed; PMID 18330697.
- Søreide JA, Lea OA, Anda O, Skarstein A, Varhaug JE, Kvinnsland S. Progesterone-binding cyst protein (PBCP) in operable breast cancer: Correlations with prognostic factors and predictive value for effect of adjuvant tamoxifen treatment. Anticancer Res 1991; 11: 601–5
- Gundersen S, Hannisdal E, Søreide JA, Skarstein A, Varhaug JE. Adjuvant tamoxifen for pre- and postmenopausal women with estrogen receptor positive, node positive breast cancer: A randomized study. Breast Cancer Res Treat 1995; 36: 49–53
- Tavassoli, FA, Devilee, P. Pathology and genetics of tumours of the breast and female genital organs. In: World Health Organization Classification of Tumours, Lyon: IARC Press; 2003. p 9–112.
- Sobin, LH, Wittekind, C. Breast tumors. In: TNM classifications of malignant tumors. 6th ed. International Union Against Cancer (UICC). Hoboken, New Jersey: John Wiley & Sons; 2002. p 131–142.
- Baak JP, van Diest PJ, Voorhorst FJ, van der Wall E, Beex LV, Vermorken JB, et al. Prospective multicenter validation of the independent prognostic value of the mitotic activity index in lymph node-negative breast cancer patients younger than 55 years. J Clin Oncol 2005; 23: 5993–6001
- Søiland H, Skaland I, Janssen EA, Kørner H, Varhaug JE, Søreide JA, et al. Comparison of Apolipoprotein D determination methods in breast cancer. Anticancer Res 2008; 28: 673–80
- Norwegian Breast Cancer Group (NBCG), www.nbcg.no. [updated February 1 2008; cited May 6 2008]. Available from:, http://www.nbcg.no/nbcg.blaaboka.html#Anchor-13-37516.
- Diez-Itza I, Vizoso F, Merino AM, Sanchez LM, Tolivia J, Fernandez J, et al. Expression and prognostic significance of Apolipoprotein D in breast cancer. Am J Pathol 1994; 144: 310–20
- Do Carmo S, Seguin D, Milne R, Rassart E. Modulation of Apolipoprotein D and Apolipoprotein E mRNA expression by growth arrest and identification of key elements in the promoter. J Biol Chem 2002; 277: 5514–23
- Herynk MH, Parra I, Cui Y, Beyer A, Wu MF, Hilsenbeck SG, et al. Association between the estrogen receptor alpha A908G mutation and outcomes in invasive breast cancer. Clin Cancer Res 2007; 13: 3235–43
- Harrell JC, Dye WW, Harvell DM, Pinto M, Jedlicka P, Sartorius CA, et al. Estrogen insensitivity in a model of estrogen receptor positive breast cancer lymph node metastasis. Cancer Res 2007; 67: 10582–91
- Zilli, M, Grassadonia, A, Tinari, N, Di Giacobbe, A, Gildetti, S, Giampirtro, J, , et al. Molecular mechanisms of endocrine resistance and their implication in the therapy of breast cancer. Biochim Biophys Acta 2008. E-pub: PMID:18804516.
- Gjerde J, Kisanga ER, Hauglid M, Holm PI, Mellgren G, Lien EA. Identification and quantification of tamoxifen and four metabolites in serum by liquid chromatography-tandem mass spectrometry. J Chromatogr A 2005; 1082: 6–14
