Abstract
Introduction. The purpose of this study was to evaluate the association between response at recurrence to letrozole versus tamoxifen and the expression of estrogen regulated proteins individually and combined in an “ER activity profile” in primary tumor tissue. Our hypothesis is that letrozole may be more effective than tamoxifen for treatment of tumors with high intratumoral estrogen content, whereas tamoxifen may be more efficient for treatment of tumors with high levels of the estrogen receptor (ER) and low intratumoral estrogen content. Materials and methods. For this study, we produced tissue microarrays from formalin fixed paraffin embedded primary tumor material from a subgroup of patients (9.4%), who have participated in the international, randomized, phase III clinical trial PO25 comparing letrozole with tamoxifen in 907 patients with advanced breast cancer. The expression levels of ER, the progesterone receptor (PR), the anti-apoptotic protein Bcl-2 and the Insulin like Growth Factor Receptor I (IGF-IR) were determined by semi-quantitative immunohistochemistry. Results. Response to letrozole and tamoxifen treatment, measured as time to progression (TTP), was independent of primary tumor expression level of ER, Bcl-2 and IGF-IR. However, high expression of PR as well as high expression of three different ER activity profiles; ER/PR/Bcl-2, ER/PR/IGF-IR and ER/PR/Bcl-2/IGF-IR identified letrozole treated patients with significantly longer TTP. The ER activity profile including ER, PR, Bcl-2 and IGF-IR showed a trend towards being a selection criterion for letrozole versus tamoxifen therapy. Discussion. This small sub-study supports our hypothesis that letrozole is superior to tamoxifen primarily in patients expressing high levels of estrogen regulated proteins in the primary tumor tissue. Furthermore, it seems that the “ER activity profile” with high PR, IGF-IR and Bcl-2 is a promising selection criterion, regarding prediction of response to letrozole versus tamoxifen.
Breast cancer is the most frequent malignancy among women in the western world, and approximately one in ten women will develop breast cancer. Estrogen plays a central role in the development and growth of the majority of breast cancers, and the estrogen receptor (ER) has long been known to define a group of patients, responding to hormonal therapy Citation[1]. Growth of tumors responding to endocrine therapy is driven by ER, which upon binding of ligand becomes activated and functions as a transcription factor. The ER is involved in regulation of a multitude of genes, many of which are associated with increased growth and survival Citation[2]. Targeted therapy for endocrine responsive tumors is directed to inhibit activation of ER. Thus, treatment with the antiestrogen tamoxifen, which competes with the natural ligand estradiol, has been efficient first line therapy for more than 30 years. The new third-generation aromatase inhibitors (AIs) anastrozole, exemestane and letrozole, exert profound inhibition of estradiol synthesis, and have in randomized trials been comparable or superior to tamoxifen in treatment of postmenopausal women with hormone receptor (HR) positive metastatic breast cancer Citation[3–7]. The consistent findings in the Femara Study PO25 included significant improvements in response rate, time to progression and early survival and thus provide the best evidence for the advantage of first-line treatment with an AI Citation[4], Citation[5]. However, theoretically the difference observed in the PO25 trial may be derived from a relatively large benefit in a small proportion of the ER positive patients receiving letrozole.
Although ER expression is a requirement for response to endocrine therapy, ER positivity is not a guarantee of sensitivity to treatment, as approximately one out of three patients with ER positive tumors has no benefit from endocrine therapy Citation[8]. Patients with primary tumors expressing higher levels of ER appear to have greater benefit from endocrine treatment than those with tumors having a positive ER status but low expression of ER Citation[9–12]. This seems to hold true in both the adjuvant and metastatic setting Citation[10], Citation[11], Citation[13], but the prediction of ER positive tumors with no response to endocrine treatment, as well as the prediction of benefit from antiestrogen vs. AI treatment is not clear. Improved response rates have been seen in tumors also expressing the estrogen inducible progesterone receptor (PR) in addition to ER Citation[8], Citation[11], Citation[13]. Lipton et al. have shown that in the PO25 trial, letrozole treated patients with normal, but not elevated, serum HER-2 level demonstrated a significantly better response to therapy than patients treated with tamoxifen Citation[14]. Furthermore both normal and elevated pre-treatment serum levels of TIMP-1 were shown to predict better response to letrozole as compared to tamoxifen treatment of these patients Citation[15]. However, it has been shown that neither PR nor HER-2 status of the primary tumor can be used as a selection criterion for treatment with letrozole versus tamoxifen in the adjuvant setting Citation[12], Citation[16].
In order to improve the selection of patients for endocrine therapy, and to enable better discrimination of the patients to tamoxifen versus AI treatment, we have looked for new predictive markers in primary tumor material from patients who participated in the PO25 study. We suggested that the expression level of PR in combination with the level of other proteins associated with estrogen stimulated growth, such as IGF-IR and Bcl-2 Citation[17], Citation[18], could constitute an “ER activity profile”, capable of predicting response to endocrine therapy with a greater accuracy than ER and PR. A recent study reported a multi-marker model in which ER, PR, Bcl-2 were included in a model that predicted outcome for response to tamoxifen significantly better than standard hormone receptors and clinicopathologic guidelines Citation[19]. Furthermore, in a preclinical breast cancer model system, expression of PR and IGF-IR is completely lost and Bcl-2 is down regulated in antiestrogen resistant cell lines as compared to cell lines that respond to treatment, indicating a predictive role of these proteins for antiestrogen response Citation[20]. A suitable method for evaluation of marker proteins is immunohistochemistry (IHC) with the use of Tissue Micro Arrays (TMA). TMAs allow rapid, cost-effective and standardized analyses with the use of only a small amount of paraffin embedded tumor tissue and it has made immunohistochemical characterization of protein expression in large series of tumors easier and less laborious Citation[21]. Furthermore, TMAs can be used for semi-quantitative analysis of various potential predictive marker proteins Citation[22].
It is our working hypothesis that AI treatment may be more effective than tamoxifen in patients with high intratumoral estrogen content, based on the efficient suppression of estrogen synthesis by AIs and the low potency of tamoxifen as a competitor for estrogen binding to ER Citation[23], Citation[24]. In contrast, tamoxifen may be most efficient in tumors with high ER expression and low intratumoral estrogen content measured as a low level of estrogen regulated proteins. Therefore, we have performed IHC on TMAs from primary breast tumors and tested both single markers and combinations of markers, “ER activity profiles”, which are designed to reflect the intratumoral estrogen level.
Materials and methods
Patients and tumor material
This study was derived from the PO25 study, an international, multicenter, double-blind, randomized, two-arm, phase III clinical trial comparing letrozole with tamoxifen as first-line treatment for postmenopausal women with advanced breast cancer Citation[4]. The study was conducted in accordance with the ethical principles that originated in the Helsinki II declaration and the appropriate local ethics review boards have approved the study.
For this current study it was possible to collect primary tumor material from 68 Danish patients and from 20 patients from the rest of Europe. Thus, altogether, paraffin blocks containing primary tumor material from 88 patients were collected. Sixty-five tumors were classified as invasive ductal and 12 as invasive lobular carcinoma. Three tumor samples were classified as neither lobular nor ductal and eight samples were not suitable for classification. Three of the 88 patients were originally allocated to a combination treatment of letrozole and tamoxifen. This combination therapy arm was stopped in the original PO25 study and the patients were excluded from the intention to treat analysis, thus 85 patients are included in the present study. All end points (Time To Progression (TTP), Overall Response Rate (ORR), Clinical Benefit (CB) and Over-all Survival (OS)) are defined as described by Mouridsen et al. 2001 Citation[4]. All patient data used in this study were provided by Novartis to the Danish Breast Cancer Cooperative Group (DBCG), in agreement with the departments who treated the patients.
Immunohistochemical staining
Sections, 3 µm, were dewaxed in coconut oil and rehydrated in a graded series of ethanol. Slides were preheated for 10 min. and boiled for 15 min. in microwave oven at 600 W in TEG buffer (pH = 9, Bie & Berntsen, Denmark) for antigen retrieval and rinsed in tap water. All immunostainings were performed at room temperature using the automated immunostainer Tech-mate 500 (Dako, Denmark), according to the following protocol: Slides were washed in TBS + 0.1% BRIJ-35 detergent (AX-LAB, Denmark) and incubated with primary antibody diluted in TBS + 0.1% BRIJ-35 + 1% BSA (Bovine Serum Albumin) + 15 nM sodiumazide for 60 min. After washing, endogenous peroxidase activity was blocked using 3% H2O2 in TBS + 0.1% BRIJ-35. The ChemMate EnVision+ Detection Kit (Peroxidase/Dab, Rabbit/Mouse, K5007, Dako, Denmark) was used as detection system for the primary antibodies. After washing, slides were counterstained with haematoxylin and dehydrated in graded series of ethanol, and finally mounted with Pertex (Histolab, Denmark). The following primary antibodies, all monoclonal mouse subtype IgG1, were used: ER, clone ER1D5, 1:200 (Immunotech); PR, clone 16, 1:200 (Novocastra); IGF-IR, clone 24-31, 1:200 (NeoMarkers) and Bcl-2, clone 124, 1:300 (Dako, Denmark). The specificity of the immunoreactions was verified by substitution of the primary antibody with the corresponding concentration of mouse IgG1, X 0931 (Dako, Denmark). In addition, positive control slides with breast tumor tissue or other tissue known to stain positive were included in every run. All stainings of the individual antigens were performed in a single run in order to minimize interserial staining variation.
Preparation of tissue microarrays
Tissue microarray blocks were constructed using the TMA-builder from Histopathology Ltd. (AH-diagnostics, Denmark). Targets for arraying (areas with representative invasive tumor) were identified by marking the corresponding areas on haematoxylin-eosin stained sections from each paraffin block. Two tissue cores with a diameter of 2 mm were transferred from each donor block to the recipient TMA block. Kidney tissue was placed in the first core of the upper left and right corner of the TMA block to ensure correct orientation when examining the slides. All analyses were performed with the use of TMAs, allowing semi-quantitative analysis Citation[21], Citation[22].
Evaluation of immunohistochemical staining
All specimens were evaluated without knowledge of the clinical data. TMA slides were evaluated by light microscopy and the authors KLH scored all the samples and BBR has been consulted in cases of doubt. Only invasive tumor components were considered when judging the staining. Semi-quantitative determination of ER and PR was performed according to the method described by Allred et al. Citation[25]. This method was also used for semi-quantitative determination of the non-nuclear cytoplasmatic marker Bcl-2. In brief: The proportion of positive stained cells was judged as 0 = no cells stained, 1 = between 0 and 1% stained positive, 2 = between 1 and 10% stained positive, 3 = between 10 and 33% stained positive, 4 = between 33 and 66% stained positive, 5 = between 66 and 100% stained positive. In addition to the proportion score, an intensity score was made based on the average intensity of staining, 0 = negative, 1 = weak, 2 = intermediate and 3 = strong. The intensity score and the proportion score was added to obtain the total score referred to as the Allred score, which is 0 or between 2 and 8, 0 and 2 were interpreted as negative. Only nuclear staining was judged when scoring ER and PR, whereas cytoplasmatic staining was scored for Bcl-2. IGF-IR, being a membrane-bound protein, was scored according to the guidelines for HER-2 staining as 0, 1+, 2+ or 3+. In brief: 0 = no staining or membranous staining in less than 10% of invasive tumor cells; 1 + = faint or barely perceptible incomplete membrane staining in more than 10% of invasive tumor cells; 2 + = weak to moderate complete membranous staining in more than 10% of invasive tumor cells; 3 + = strong complete membranous staining in more than 10% of invasive tumor cells. Scores 0 and 1+ were interpreted as negative in this study.
Statistical analysis
Chi-square test was used to evaluate if the 85 patients in the present study represent the entire study population of 907 patients ( and ) and p-values are given. The agreement between expression of PR with the two other estrogen regulated proteins Bcl-2 and IGF-IR was done by calculating the Kappa score and p-values are given (). Survival curves in relation to predictive markers are illustrated by Kaplan-Meier plots and difference in time to progression (TTP) is evaluated by the Wilcoxon rank sum test. For multivariate analysis, Cox-regression analysis was applied. When analysis of grouped scoring values was applied, scoring values were grouped as follows: For ER, Bcl-2 and PR expression “high” is defined as Allred score 7 or 8, “low” as Allred score 3 to 6 and “negative” as 0 or 2. For IGF-IR, “high” is defined as Herceptest score 3, “low” as Herceptest score 2, and “negative” as Herceptest score 0 and 1 (See ). For the ER activity profiles to be categorized as “high” all estrogen regulated proteins included in the profile must be of high expression (Allred 7 or 8 for PR and Bcl-2, and Herceptest score 3 for IGF-IR). The ER activity profiles are regarded as “intermediate” if all but one of the estrogen regulated proteins are expressed at a lower level than Allred 7 or 8 and Herceptest score 3, but not negative. Tumors with a lower expression of estrogen regulated proteins than this are classified as ER activity profile ”low”. All tests are two-sided and a p-value <0.05 was used as level of significance.
Table I. Patient data: Patient characteristics for the 85 patients in this study and for all 907 patients from the intent-to-treat population in the P025 trial.
Table II. Response to therapy.
Table III. Semi-quantitative IHC results.
Results
Patient data
In this study primary tumor material from 9.4% of the patients who participated in the original PO25 efficacy study by Mouridsen et al. Citation[4] are included. As in the original study, the number of patients allocated to letrozole and tamoxifen is well balanced in our subgroup of patients, 46 (54%) and 39 (46%) respectively.
outlines patient baseline characteristics before study treatment for the entire patient population of 907 patients and our subgroup of 85 patients. Age as well as KPS (Karnofsky Performance Score) are well balanced between all groups, but only 15% and 18% are stage IV at time of primary diagnosis in this current study, compared to 32% for both letrozole and tamoxifen treated patients in the original study. Accordingly, 85% and 82% of the patients in the current study have relapsing metastatic disease at study entry compared to only 68% for both letrozole and tamoxifen treated patients in the original study. Thus, the subgroup of patients from which we have been able to collect tumor material was in a significantly more advanced stage of disease at study entry than the entire study population (p = 0.0012, χ2 test). Furthermore, a significantly larger proportion of the 85 patients had received prior adjuvant treatment with tamoxifen, 33% and 26%, compared to 19% and 18% for letrozole and tamoxifen respectively in the entire study (p = 0.006, χ2 test).
outlines the efficacy end points for response to treatment. Whereas letrozole was superior to tamoxifen with respect to TTP, ORR and CB for all 907 patients, no difference in response to tamoxifen or letrozole was observed in the subgroup of 85 patients included in this current study. Median TTP was 9.5 months (90% range: 0.33–39) for letrozole and 9.5 months (90% range: 2.6–46) for tamoxifen. However, no significant overall difference was found between ORR and CB in this small patient subgroup compared to all patients. The median OS is shorter for the letrozole treated patients, 28 months (90% range: 4.7–48), compared to 38 months (90% range: 3.8–56) for tamoxifen treated patients in our small subgroup.
Scoring results of immunohistochemical staining of marker proteins
The selected markers were scored on TMAs as described in the materials and methods section. The semi-quantitative scoring results of ER, PR, Bcl-2 and IGF-IR are shown in . It can be seen that the majority of tumors had a high Allred score for ER and PR. The patients enrolled in this study had all previously been classified as receptor positive (ER and or PR positive) or unknown Citation[4], and the results from this study confirmed previous tests since no tumor was negative for both ER and PR. Only 6% of the tumors were scored negative for ER, 16% low and 68% high. The PR was more evenly distributed on the Allred scale, 21% of the tumors were scored negative, 33% low and 38% of the patients had a high expression of PR. High cytoplasmic staining of the anti-apoptotic protein Bcl-2 was observed in 64% of the samples, 16% showed low Bcl-2 expression, and only 8% were negative. Staining of the IGF-IR had the same appearance as e.g. HER-2 staining, except that IGF-IR, as previously described was also often seen in the cytoplasm Citation[22]. IGF-IR scoring values of 2+ and 3+ were observed in 28% and 32% of the samples, respectively. For all markers technical problems impaired the scoring of between 7 and 14 of the tumor samples making them “not assessable” (N.A.). For IGF-IR, most of the non assessable samples had too much cytoplasmic staining to justify scoring of the plasma membrane.
Single markers to select patients for treatment with either tamoxifen or letrozole
To evaluate whether our analyses of ER, PR, Bcl-2 or IGF-IR could be used to discriminate between time to progression (TTP) for patients treated with tamoxifen or letrozole, we compared TTP for all patients separated in subgroups of negative, low or high marker expression (as described in material and methods), as well as between letrozole and tamoxifen treatment. For each marker, TTP was compared by uni-variate Wilcoxon-test, and Kaplan-Meier plots as shown for ER and PR in . For ER no significant difference was found between any of the marker expression levels, or the two different endocrine therapies, indicating that the expression level of ER (A) was not found to be predictive of TTP for letrozole or tamoxifen treatment (p = 0.88). When patients were separated according to PR expression status (B), a significant difference between TTP was found (p=0.0093), and a Cox analysis disclosed a significant difference between the effect of endocrine treatment in patients with negative, low and high PR expression (p = 0.0067). A further subgroup analysis of PR expression for letrozole treated patients with ER positive tumors revealed a significantly different TTP for letrozole treated patients with negative (median TTP 4.5 months (90% range: 0–24)), low (median TTP 8.0 months (90% range: 0–15)) and high (median TTP 16.5 months (90% range: 0–44)) PR expression in the primary tumor, (A, p = 0.042). No difference in TTP was observed for patients who received tamoxifen treatment (p = 0.24, data not shown). When looked upon alone, neither of the other two estrogen regulated proteins, Bcl-2 or IGF-IR turned out to be of significance for TTP and treatment, p = 0.50 and p = 0.25 respectively.
Figure 1. Time to progression (TTP) by high, low and negative expression of ER and PR in primary tumor tissue for patients treated with either letrozole (black) or tamoxifen (grey). (A) TTP by ER expression: Letrozole treatment, ER high, n = 31; ER low, n = 9; ER negative, n = 3. Tamoxifen treatment, ER high, n = 27; ER low, n = 5; ER negative, n = 2, p = 0.88. (B) TTP by PR expression: Letrozole treatment, PR high, n = 20; PR low, n = 13; PR negative, n = 9. Tamoxifen treatment, PR high, n = 12; PR low, n = 15; PR negative, n = 9, p = 0.0093.
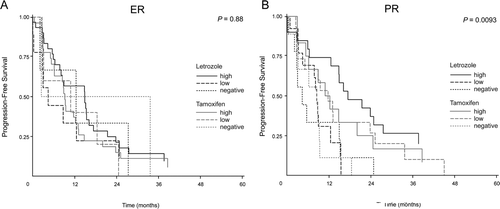
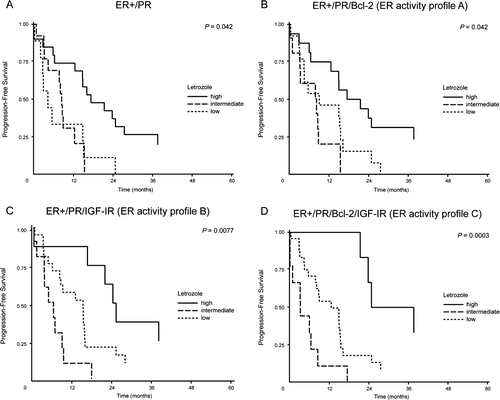
Figure 2. Time to progression (TTP) in letrozole treated patients with ER positive primary tumors by high, low and negative expression of PR, ER activity profile A, ER activity profile B and ER activity profile C. (A) TTP by PR: Letrozole treatment, high, n = 20; intermediate, n = 12; low, n = 8, p=0.042 (B) TTP by ER activity profile A: Letrozole treatment, high, n = 16; intermediate, n = 11; low, n = 13, p=0.042. (C) TTP by ER activity profile B: Letrozole treatment, high, n = 8; intermediate, n = 11; low, n = 21, p=0.0077. (D) TTP by ER activity profile C: Letrozole treatment, high, n = 6; intermediate, n = 9; low, n = 25, p=0.0003.
Marker combinations to select patients for treatment with either tamoxifen or letrozole: “ER activity profiles”
PR, Bcl-2 and IGF-IR are known to be estrogen regulated proteins. We compared the expression level of PR with Bcl-2 and IGF-IR by kappa statistics (), and found that Bcl-2, but not IGF-IR expression level, was significantly associated with the expression level of PR. Thus, Bcl-2 expression may not add extra information to the profile than PR, whereas IGF-IR may give additional value. To evaluate our hypothesis, we looked at the expression level of PR (negative, low and high) in ER positive samples in relation to TTP, and tested three “ER activity profiles” for the ER positive samples: One that included PR and Bcl-2; “ER activity profile A”; one that included PR and IGF-IR “ER activity profile B”, and one with PR, Bcl-2 and IGF-IR “ER activity profile C”. All profiles were divided into high, intermediate and low, as described in materials and methods.
Table IV. Association between the expression level of PR, IGF-IR and Bcl-2.
When looking at all patients (letrozole and tamoxifen treated patients together), Cox analysis disclosed significant difference between TTP for patients with low, intermediate and high ER activity profile expression. This was the case for all three “ER activity profiles” with p = 0.015, p = 0.033 and p = 0.018 for profile A, profile B and profile C, respectively. Further subgroup analysis of low, intermediate and high profile expression for letrozole treated patients disclosed a significantly different TTP for “ER activity profile A” which included both PR and Bcl-2 (B), (p = 0.042). With the addition of IGF-IR instead of Bcl-2 in “ER activity profile B”, the level of significance was increased, C, p = 0.0077 and addition of both Bcl-2 and IGF-IR to the profile in “ER activity profile C”, increased the level of significance further, D, p = 0.0003. For tamoxifen treated patients neither low, nor intermediate or high expression of any of the profiles revealed a difference in TTP.
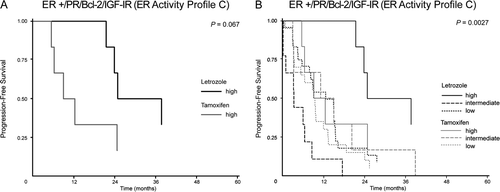
The primary goal of this study was to find a single marker or marker profile being useful in selection of patients who will benefit most from treatment with tamoxifen or letrozole, respectively. We found a trend indicating that high expression of “ER activity profile C” could be a selection criterion for treatment with letrozole versus tamoxifen (p = 0.067, A), with a median TTP of 25 months for letrozole treatment versus 9 months for tamoxifen treatment. When looking at all patients together, letrozole treated patients with high expression of “ER activity profile C” differs from the rest of the patients (p = 0.0027, B).
Figure 3. Time to progression (TTP) by ER activity profile C in patients treated with letrozole or tamoxifen. (A) TTP by high ER activity profile C: letrozole treatment (black), median TTP 31 months (90% range: 21–50), n = 6, tamoxifen treatment (grey) median TTP 10 months (90% range: 5–45), n = 6; p = 0.067. (B) TTP by ER activity profile C (all patients): Letrozole treatment (black), high, n = 6; intermediate, n = 9; low, n = 25, tamoxifen treatment (grey), high, n = 6; intermediate, n = 6; low, n = 20, p=0.0027.
Discussion
Breast cancer is a heterogeneous disease and cancer progression is a complex process, involving pathways that are controlled by hormones, growth factors and other signaling molecules. Improved classification of the subtypes of breast cancer will hopefully result in higher response rates to the different types of treatment. Gene expression analysis has disclosed at least five different subtypes Citation[26], but this method is not at present routine. Therefore, still a potential classification by means of IHC analysis will be an advantage. Ring et al. Citation[27] reported the use of a panel of five antibodies for prognostic use in patients with ER positive tumors. In ER positive breast cancer, estrogen regulates expression of a large number of genes and corresponding proteins, and among these are the PR, IGF-IR and Bcl-2, which in this study have been selected as potential markers for intratumoral estrogen level. Our working hypothesis is that AI treatment will be more efficient than tamoxifen in patients with high intratumoral estrogen content, as the AI suppresses the estrogen level to nearly undetectable level Citation[23], whereas tamoxifen may not be potent enough as a competitor in tumors with high intracellular estrogen level Citation[24]. It may be speculated that tamoxifen is most efficient in tumors with high ER level and low levels of estrogen regulated proteins.
The patients in our study were equally distributed with respect to letrozole or tamoxifen therapy, but in contrast to the original study, we did not find a superior response to letrozole in this small patient population. Our aim was to investigate whether single markers or marker combinations could be used to select patients for treatment with either tamoxifen or letrozole. None of our single markers or our ER activity profiles were able to select groups of patients with superior benefit from tamoxifen therapy. For letrozole treatment, patients with ER positive tumor and high PR expression had significantly longer progression free survival (p = 0.042), indicating that the effect of letrozole treatment in patients with advanced disease may be predicted from the PR expression level in the primary tumor tissue. By addition of more estrogen regulated proteins in an estrogen response profile, we could demonstrate that addition of IGF-IR, but not Bcl-2, improved selection of a group of patients with long progression free survival. The lack of additional value by adding Bcl-2 may be explained by the correlation between PR and Bcl-2 expression. In agreement, the ER profile with PR, IGF-IR and Bcl-2 expression was only slightly better than the profile that included only PR and IGF-IR. Altogether, subgroup analyses showed that letrozole treated patients with high PR, high “ER activity profile A”, high “ER activity profile B”-or high “ER activity profile C” expression in the primary tumor, experience longer median TTP compared to patients with less expression of these marker profiles. A median TTP of 25 months was seen in patients with high PR and IGF-IR expression as well as in patients with high PR, IGF-IR and Bcl-2 expression. All other groups of patients had a median TTP less than 15 months. Thus expression of two or three estrogen regulated proteins selects a group of patients who experience a long duration of response to letrozole therapy. These data confirm our hypothesis that the greatest benefit from AI treatment is seen in patients with a high expression level of estrogen regulated proteins, reflecting a high intratumoral estrogen content and ER driven cell growth.
In our study an ER activity profile with high PR, IGF-IR and Bcl-2 expression selected a group of patients with superior response to letrozole and although the analysis did not reach statistical significance, p = 0.067, this profile deserves to be tested in a larger set of patients.
Viale et al. have shown that PR expression levels above 1% do not increase DFS of letrozole treated patients in the adjuvant setting Citation[12]. In contrast to this, we found that in patients with metastatic disease, high PR level may be relevant as predictive marker to select between a longer or shorter TTP with letrozole treatment. An explanation may be that in the adjuvant BIG 1-98 study, patients who experienced an early relapse may, for a large part, be patients with estrogen independent tumors in spite of HR expression. The finding that high PR expression in the present study of patients with advanced breast cancer predicts a longer TTP with letrozole treatment is in accordance with the hypothesis that patients with tumors that have a high PR expression may benefit more from removal of estrogen with an AI. This hypothesis is further supported by the finding in this present study that patients with tumors expressing high levels of PR and IGF-IR or high PR, IGF-IR and Bcl-2 level experience significantly longer TTP than the rest of the patients. These data also support our hypothesis that tamoxifen may not be potent enough to compete with high intracellular estradiol content, and these patients should not be treated with tamoxifen but with an AI or a more potent antiestrogen, such as fulvestrant. Thus for letrozole treated patients IHC analysis of ER in combination with semi-quantitative analysis of PR, Bcl-2 and IGF-1R will enable a better selection of patients, who benefit from treatment.
The small sample size of the current sub-study prohibits any firm conclusions, but the present data support our hypothesis that superiority of AI treatment to tamoxifen treatment may primarily arise from the beneficial effect of AI treatment in the subgroup of patients expressing high levels of estrogen regulated proteins in the primary tumor tissue. Furthermore, it seems that in our current pilot study, the “ER activity profile C” with high PR, IGF-IR and Bcl-2 is a promising selection criterion, regarding prediction of response to letrozole versus tamoxifen. Additional experiments are in progress to test the profile in the adjuvant setting.
Acknowledgements
We kindly acknowledge the participating Danish and European Pathology Departments for supplying paraffin embedded tumor material: Aalborg Sygehus, Bispebjerg Hospital, Hjørring Sygehus, Hvidovre Hospital, Nykøbing Falster Sygehus, Odense Universitetshospital, Rigshospitalet, Roskilde Amts Sygehus, Slagelse Sygehus, Svendborg Sygehus, Sønderborg Sygehus, Vejle Sygehus, Viborg Sygehus, J.W. Goethe-Universität, Universitätsklinikum Hamburg-Eppendorf, Centre Regional Francois Baclesse, Centre Hospitalier Général André-Boulloche, Kuopio University Hospital and Turku University Hospital. Birgit S. Hansen, Annegrethe Schaadt, Vinni Bredahl and Heidi Frogne are gratefully acknowledged for excellent technical assistance. This study was supported financially by Danish Cancer Society, “Fonden til fremme af klinisk eksperimentiel cancerforskning specielt vedrørende cancer mammae” and “Lykfeldt legatet”. The authors declare that they have no competing interests.
References
- Mouridsen H, Palshof T, Patterson J, Battersby L. Tamoxifen in advanced breast cancer. Cancer Treat Rev 1978; 5: 131–41
- Frasor J, Danes JM, Komm B, Chang KC, Lyttle CR, Katzenellenbogen BS. Profiling of estrogen up- and down-regulated gene expression in human breast cancer cells: Insights into gene networks and pathways underlying estrogenic control of proliferation and cell phenotype. Endocrinology 2003; 144: 4562–74
- Bonneterre J, Buzdar A, Nabholtz JM, Robertson JF, Thurlimann B, von EM, et al. Anastrozole is superior to tamoxifen as first-line therapy in hormone receptor positive advanced breast carcinoma. Cancer 2001; 92: 2247–58
- Mouridsen H, Gershanovich M, Sun Y, Perez-Carrion R, Boni C, Monnier A, et al. Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: Results of a phase III study of the International Letrozole Breast Cancer Group. J Clin Oncol 2001; 19: 2596–606
- Mouridsen H, Gershanovich M, Sun Y, Perez-Carrion R, Boni C, Monnier A, et al. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: Analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol 2003; 21: 2101–9
- Nabholtz JM, Buzdar A, Pollak M, Harwin W, Burton G, Mangalik A, et al. Anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: Results of a North American multicenter randomized trial. Arimidex Study Group. J Clin Oncol 2000; 18: 3758–67
- Wardley A. The need for advanced breast cancer treatment guidelines: Results of an internet-based survey. Breast 2008; 17: 275–81
- Osborne CK, Yochmowitz MG, Knight WAIII, McGuire WL. The value of estrogen and progesterone receptors in the treatment of breast cancer. Cancer 1980; 46: 2884–8
- Early Breast Cancer Trialists' Collaborative Group. Tamoxifen for early breast cancer: An overview of the randomised trials. Lancet. 1998;351:1451–67.
- Elledge RM, Green S, Pugh R, Allred DC, Clark GM, Hill J, et al. Estrogen receptor (ER) and progesterone receptor (PgR), by ligand-binding assay compared with ER, PgR and pS2, by immuno-histochemistry in predicting response to tamoxifen in metastatic breast cancer: A Southwest Oncology Group Study. Int J Cancer 1997; 89: 111–7
- Bardou VJ, Arpino G, Elledge RM, Osborne CK, Clark GM. Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases. J Clin Oncol 2003; 21: 1973–9
- Viale G, Regan MM, Maiorano E, Mastropasqua MG, Dell'Orto P, Rasmussen BB, et al. Prognostic and predictive value of centrally reviewed expression of estrogen and progesterone receptors in a randomized trial comparing letrozole and tamoxifen adjuvant therapy for postmenopausal early breast cancer: BIG 1–98. J Clin Oncol 2007; 25: 3846–52
- Ravdin PM, Green S, Dorr TM, McGuire WL, Fabian C, Pugh RP, et al. Prognostic significance of progesterone receptor levels in estrogen receptor-positive patients with metastatic breast cancer treated with tamoxifen: Results of a prospective Southwest Oncology Group study. J Clin Oncol 1992; 10: 1284–91
- Lipton A, Ali SM, Leitzel K, Demers L, Harvey HA, Chaudri-Ross HA, et al. Serum HER-2/neu and response to the aromatase inhibitor letrozole versus tamoxifen. J Clin Oncol 2003; 21: 1967–72
- Lipton A, Leitzel K, Chaudri-Ross HA, Evans DB, Ali SM, Demers L, et al. Serum TIMP-1 and response to the aromatase inhibitor letrozole versus tamoxifen in metastatic breast cancer. J Clin Oncol 2008; 26: 2653–8
- Rasmussen BB, Regan MM, Lykkesfeldt AE, Dell'Orto P, Del CB, Henriksen KL, et al. Adjuvant letrozole versus tamoxifen according to centrally-assessed ERBB2 status for postmenopausal women with endocrine-responsive early breast cancer: Supplementary results from the BIG 1–98 randomised trial. Lancet Oncol 2008; 9: 23–8
- Elledge RM, Green S, Howes L, Clark GM, Berardo M, Allred DC, et al. bcl-2, p53, and response to tamoxifen in estrogen receptor-positive metastatic breast cancer: A Southwest Oncology Group study. J Clin Oncol 1997; 15: 1916–22
- Peyrat JP, Bonneterre J, Beuscart R, Djiane J, Demaille A. Insulin-like growth factor 1 receptors in human breast cancer and their relation to estradiol and progesterone receptors. Cancer Res 1988; 48: 6429–33
- Linke SP, Bremer TM, Herold CD, Sauter G, Diamond C. A multimarker model to predict outcome in tamoxifen-treated breast cancer patients. Clin Cancer Res 2006; 12: 1175–83
- Lykkesfeldt AE, Madsen MW, Briand P. Altered expression of estrogen-regulated genes in a tamoxifen-resistant and ICI 164,384 and ICI 182,780 sensitive human breast cancer cell line, MCF-7/TAMR-1. Cancer Res 1994; 54: 1587–95
- Kyndi M, Sørensen FB, Knudsen H, Overgaard M, Nielsen HM, Andersen J, et al. Tissue microarrays compared with whole sections and biochemical analysis. A subgroup analysis of DBCG 82 b&c. Acta Oncol 2008; 47: 591–9
- Henriksen KL, Rasmussen BB, Lykkesfeldt AE, Moller S, Ejlertsen B, Mouridsen HT. Semi-quantitative scoring of potentially predictive markers for endocrine treatment of breast cancer: A comparison between whole sections and tissue microarrays. J Clin Pathol 2007; 60: 397–404
- Bhatnagar AS. The discovery and mechanism of action of letrozole. Breast Cancer Res Treat 2007; 105(Suppl 1)7–17
- Nicholson RI, Syne JS, Daniel CP, Griffiths K. The binding of tamoxifen to oestrogen receptor proteins under equilibrium and non-equilibrium conditions. Eur J Cancer 1979; 15: 317–29
- Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol 1998; 11: 155–68
- Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 2001; 98: 10869–74
- Ring BZ, Seitz RS, Beck R, Shasteen WJ, Tarr SM, Cheang MC, et al. Novel prognostic immunohistochemical biomarker panel for estrogen receptor-positive breast cancer. J Clin Oncol 2006; 24: 3039–47
