Abstract
Purpose. Continuous minor steps of improvement in the management of breast cancer have resulted in decreased mortality rates during the last decades. The aim of this study was to compare the clinical outcome of patients with stage I breast cancer diagnosed during two time periods that differed with respect to adjuvant systemic therapy. Material and methods. The studied population consisted of all women < 60 years of age, who were diagnosed breast cancer stage I between 1986 and 1999 in south-east Sweden, a total of 1 407 cases. The cohort was divided into two groups based on the management programmes of 1986 and 1992, hereafter referred to as Period 1 and Period 2. Before 1992 the only adjuvant systemic therapy recommended was tamoxifen for hormone receptor positive patients aged 50 years or older. During Period 2 the use of adjuvant treatment was extended to younger patients at high risk, identified by a high tumour S-phase fraction, with either hormonal or cytotoxic treatment. Results. The estimated distant recurrence-free survival rate was significantly higher during Period 2 than during Period 1 (p = 0.008). Subgroup analysis showed that the most evident reduction of distant recurrence risk was among hormone receptor-negative patients (HR = 0.58, 95% CI 0.31–1.09, p = 0.09) and among patients with a high tumour S-phase fraction (HR = 0.53, 0.30–0.93, p = 0.028). The risk reduction between the periods was still statistically significant in multivariate analysis when adjusting for different tumour characteristics and treatment modalities, indicating an influence of other factors not controlled for. One such factor may be the duration of tamoxifen treatment, which likely was more frequently five years during Period 2 than during Period 1. Conclusions. We conclude that the causes of the increase in distant recurrence free survival for women with breast cancer stage I are complex. The results support though that high-risk subgroups of stage I breast cancer patients did benefit from increased use of systemic therapy as a consequence of an updated management programme.
Breast cancer incidence rates have increased among women in Europe and in the USA during the last decades and it is the most common type of cancer among women Citation[1–3]. In Sweden, the yearly incidence and prevalence are high, however by international comparison, the Swedish breast cancer mortality rates have been low Citation[4]. A recent population based study from south-east Sweden presented breast cancer specific ten-year survival rates of 90.9% for stage I disease (T1N0M0) and 74.0% for all stages Citation[5]. A national screening schedule for women aged 40–74 years introduced in 1986, changes in surgery towards breast-conserving surgery in combination with adjuvant radiotherapy as opposed to mastectomy, more extensive use and tailoring of systemic adjuvant treatment for breast cancer stage I since the early 1990s might have been of importance for the high survival rates.
The prognosis of breast cancer patients is favourable if the disease is detected and adequately treated at an early stage Citation[5], Citation[6]. Estimation of recurrence risk for breast cancer stage I is based on malignancy grade, tumour proliferation activity, steroid hormone receptor status and tumour size Citation[6]. A high S-phase fraction (SPF), or a high Nottingham histological grading (NHG) score indicate high risk of distant recurrence Citation[6–11]. Tumour expression of oestrogen and/or progesterone receptors indicates a better short-term prognosis compared to tumours not expressing receptors, hereafter referred to as receptor positive and receptor negative tumours respectively. Patients with smaller tumours have better prognosis than those with larger ones Citation[6], Citation[12].
Since the mid 1980s, breast cancer patients in south-east Sweden have been treated and followed within the framework of a regional management programme with guidelines for systemic adjuvant therapy based on age and recurrence risk. The first regional management programme that recommended systemic therapy for breast cancer stage I was issued in 1983 () Citation[13]. The programme was updated in 1992 with recommendations for more extensive use of hormonal therapy, and with the introduction of cytotoxic therapy () Citation[14]. The change in recommendation of adjuvant therapy in 1992 was based on clinical randomised trials that showed significantly reduced risk of recurrence and overall mortality for women with early breast cancer when given postoperative systemic therapy. Women with receptor positive tumours were shown to benefit from hormonal therapy and women with tumours with high proliferation markers benefited from cytotoxic therapy Citation[15]. Initially, the regional management programme for south-east Sweden recommended two years hormonal therapy with tamoxifen for patients with receptor positive tumours Citation[13], Citation[14]. During 1995 the recommendation was changed to five years tamoxifen, since survival rates were shown to increase with longer duration of treatment Citation[16]. The cytotoxic therapy for women with high S-phase fraction tumours initially combined cyclophosphamide, methotrexate and 5-fluoruracil (CMF), but methotrexate was gradually substituted with epirubicin (CEF) Citation[6], Citation[14] after a randomised trial between 1990 and 1998 Citation[17]. Postoperative radiotherapy was only recommended after breast conserving surgery.
Table Ia. Breast cancer stage I. Recommendations for postoperative adjuvant systemic therapy according to the management programme of 1983.
Table Ib. Breast cancer stage I. Recommendations for postoperative adjuvant systemic therapy according to the management programme of 1992.
The results from the randomised trials of systemic adjuvant treatment versus no treatment seem indisputable, but there are few data confirming this outcome in clinical practice. The conditions for the general population of unselected patients could be different than those for patients selected in randomised clinical trials. Furthermore, in the present study the selection of patients who were recommended adjuvant systemic therapy was based on prognostic indicators. The survival rates for all stages of breast cancer in south-east Sweden have been reviewed Citation[5], but the therapeutic benefits of the change in regional recommendations of systemic adjuvant therapy for stage I disease has not been studied. The main objective of this study was to evaluate if there were any changes in distant recurrence free survival and overall survival among women treated for breast cancer stage I, after the regional management programme was updated in 1992. The secondary objective was to evaluate if any changes in distant recurrence free survival could be shown in subgroups of patients, based on S-phase fraction, hormone receptor status and tumour size.
Material and methods
The study was designed as a retrospective cohort study based on data registered in the south-east Sweden regional database for breast cancer patients. Registration is compulsory, and the database holds information on surgery, lymph node involvement, tumour size, hormone receptor status, S-phase fraction, adjuvant therapy and clinical follow-up data. The studied population consisted of all women < 60 years of age, who were diagnosed breast cancer stage I between January 1986 and December 1999 in south-east Sweden, a total of 1 407 cases. The study did not include women 60 years or older, since the recommendations for systemic adjuvant treatment in practice were limited to patients younger than 60 years. The cohort was divided into two primary groups based on the management programmes of 1983 and 1992, hereafter referred to as Period 1 and Period 2. Period 1 consisted of 519 women, diagnosed 1986 through 1991. Period 2 consisted of 888 women, diagnosed 1992 through 1999. In the analysis, Period 1 had the function of a historical control group for Period 2. Each group was further divided into secondary groups based on the prognostic parameters tumour size (≤10 mm or 11–20 mm), steroid hormone receptor status (positive (≥30 fmol/µg DNA) or negative) and S-phase fraction (high (≥10%) or low (<10%)). Oestrogen and progesterone receptor concentrations were measured with enzyme immuno assays (Abbott Laboratiories, Chicago, USA) and the S-phase fraction was obtained from DNA flow cytometry analysis Citation[18]. The primary groups were analysed for overall survival and distant recurrence free survival. The secondary groups were analysed for distant recurrence free survival.
The end date of follow-up was December 31, 2006. Fifty-six women had moved from the region or had data missing because of other reasons, and were controlled at the tax office for deaths. For seven women who had left Sweden, the date of migration was used as end date. For 14 women for whom the cause of death was breast cancer, there was no data of distant recurrence and for these patients the time of death has served as time for distant recurrence. Median duration of follow-up for recurrence free patients was 17.5 years in Period 1, and 10.3 years in Period 2.
In Period 2, the patient records for 115 women with high S-phase fraction tumours, and the records for 125 women with unknown or low S-phase fraction tumours were controlled for data on cytotoxic therapy and distant recurrence. The purpose of the control was to examine whether there were any cases with adjuvant therapy and/or distant recurrence not registered in the database. According to records, four patients with high S-phase fraction tumours and three patients with low S-phase fraction tumours had received cytotoxic therapy, which had not been registered in the regional database. Three patients with high S-phase fraction tumours and three patients with low S-phase fraction tumours had developed distant recurrence, which had not been registered. These findings were taken into account before analysis of data.
Statistical methods
Kaplan-Meier survival analysis Citation[19] with log rank test Citation[20] was used for analysing overall survival and distant recurrence free survival. Cox regression analysis Citation[21] was used for analysing relative risk and for multivariate analysis. A two-sided p-value of < 0.05 was used as significance level. Relative risk, five-year and ten-year overall survival and distant recurrence free survival are stated with 95% confidence interval (CI 95%). The software used for the statistical tests was SPSS for Windows version 15.0. The figures were done with Stata/SE 10.0.
Results
Patient age and tumour characteristics
The patient median age was 50 years (range 24–59). The women in Period 2 were significantly older compared to Period 1 (p < 0.001). shows distribution of tumour characteristics divided by age and diagnosis period. S-phase fraction, receptor status, and tumour size did not differ significantly between the periods although there was a tendency towards a higher frequency of receptor positive tumours in Period 2 compared to Period 1 (p = 0.088).
Table II. Patient and tumour data in the two cohorts, also divided by age
Differences in treatment between Period 1 and Period 2
shows treatment for Period 1 and Period 2 divided by age. A significantly larger proportion of women (p < 0.001) were operated with breast-conserving surgery in Period 2 (), the change being most notable for patients with tumours 11–20 mm (data not shown). The increased breast-conserving surgery paralleled an increase of patients given adjuvant radiotherapy, in agreement with regional guidelines Citation[13], Citation[14]. However, the number of women treated with radiotherapy did slightly exceed the number operated with breast-conserving surgery.
shows frequency of hormonal and cytotoxic therapy divided by receptor status and S-phase fraction in Period 1 and 2. The frequency of patients who received hormonal therapy was significantly higher (p = 0.011) in Period 2 compared to Period 1 ( and ). However, it was only among women < 50 years that hormonal therapy increased. In the age group 50–59 years, the frequency of patients who received hormonal therapy was lower in Period 2 compared to Period 1, the main reason being a lower frequency of women with receptor negative tumours being treated with tamoxifen. The frequency of patients who received cytotoxic therapy increased during Period 2 (p < 0.001), especially among those < 50 years ( and ). Overall, the increased use of systemic therapy was largely confined to patients with high SPF tumours, in accord with the management programme recommendations.
Table III. Frequency of adjuvant systemic treatment in Period 1 and 2, divided by receptor status and S-phase fraction.
Overall survival
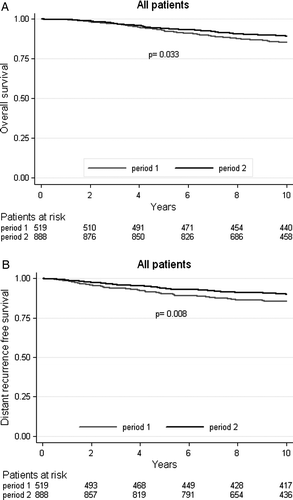
There was a 28% (HR = 0.72, CI 95% 0.53–0.98, p = 0.033) reduction of ten-year overall mortality risk in Period 2 compared to Period 1 and the overall survival after ten years increased from 85.3% in Period 1 to 89.3% in Period 2 (A).
Distant recurrence free survival
Five-year and ten-year differences in distant recurrence free survival are presented in . For all women, there was a 34% (HR = 0.66, CI 95% 0.48–0.90, p = 0.008) reduction of distant recurrence risk after ten years in Period 2 compared to Period 1 (B). The estimated absolute increase in distant recurrence free survival after ten years was 4.6% ().
Table IV. Five-year and ten-year distant recurrence free survival rates in Period 1 and 2, divided by tumour characteristics.
Multivariate analysis of distant recurrence free survival, including period of diagnosis, tumour characteristics, treatment and patient age, showed that diagnosis period remained significant after adjustment for other factors (). Among other variables, S-phase fraction was the only factor that had significant influence on the outcome. Moreover, for patients who did not receive any systemic adjuvant treatment, S-phase fraction was a strong indicator (HR = 3.25, CI 95% 1.90–5.57, p < 0.001) of distant recurrence ().
Figure 2. Distant recurrence-free survival in relation to S-phase fraction for patients given no adjuvant systemic therapy.
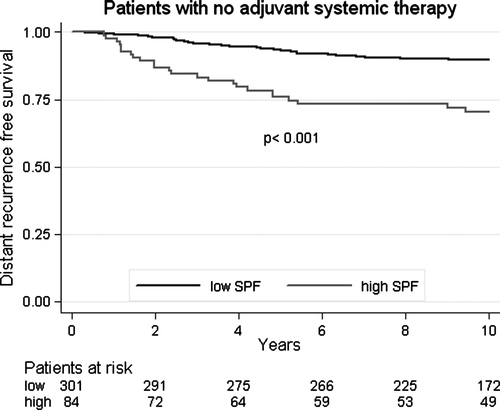
Table V. Multivariate analysis of distant recurrence rates with Cox proportional hazard method including diagnosis period, tumour characteristics, treatment and patient's age.
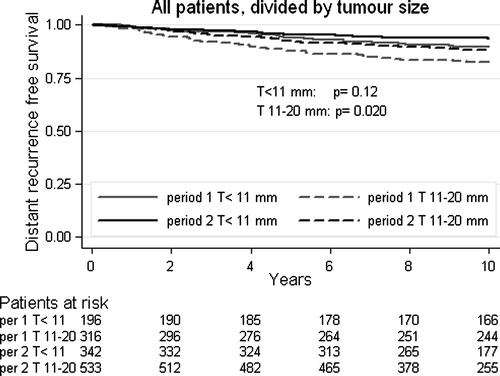
When specifically controlled for tumour size, the difference in distant recurrence risk between Period 1 and 2 was not statistically significant (HR = 0.62, CI 95% 0.34–1.15, p = 0.12) for patients with tumours ≤10 mm (). For patients with tumours 11–20 mm there was a 35% (HR = 0.65, CI 95% 0.45–0.94, p = 0.020) reduction of distant recurrence risk in Period 2 compared to Period 1 () and an estimated 5.5% absolute increase in distant recurrence free survival after ten years ().
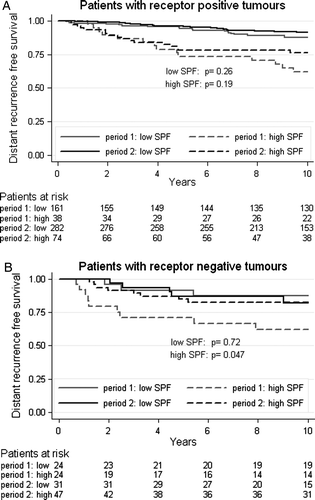
For patients with receptor positive tumours there was no statistically significant difference in distant recurrence free survival between the two periods (HR = 0.77, CI 95% 0.51–1.17, p = 0.22), while there was a 42% reduction of distant recurrence risk (HR = 0.58, CI 95% 0.31–1.09, p = 0.090) for patients with receptor negative tumours (). Furthermore, the reduction in distant recurrence rate between the periods appeared to be larger for patients with high S-phase tumours (HR = 0.53, CI 95% 0.30–0.93, p = 0.028) than for those showing a low SPF (HR = 0.77, CI 95% 0.44–1.33, p = 0.34). The estimated absolute increase in distant recurrence free survival after 10 years was 16.6% and 2.6%, respectively (). Analysis of hormone receptor status and S-phase fraction in combination revealed that the most evident difference between the periods was seen among receptor negative patients with high SPF tumours (, ).
Discussion
In this study we identified all patients < 60 years of age, who were registered for breast cancer stage I between 1986 and 1999 in the south-east Sweden regional breast cancer database. The tumour characteristics in the studied population were comparable, and the only significant difference between the studied time periods was the significantly higher frequency of women 50–59 years in Period 2 compared to Period 1. A possible contributing factor could be the increasing use of hormonal replacement therapy (HRT) among menopausal women during the 1990s. The HRT-frequency increased from < 10% during the 1980s, to > 40% in the 1990s Citation[22–24], and has been shown to increase the risk of breast cancer Citation[25].
The beneficial effect of adjuvant hormonal and cytotoxic therapy for early stages of breast cancer is known Citation[15]. By analogy with the intentions of the recommended extended use of systemic adjuvant therapy in the south-east Sweden regional management programme of 1992 Citation[14], our study has shown that for women diagnosed breast cancer stage I in Period 2, both overall survival and distant recurrence free survival have increased significantly. In agreement with the programme of 1992, the women with high S-phase tumours in Period 2 more frequently received hormonal therapy and cytotoxic therapy compared to the women in Period 1. However, the compliance to the recommendations was moderate and more patients could have received chemotherapy. The women 50–59 years of age did receive less hormonal therapy in Period 2, but since this mainly was due to a more selective approach depending on receptor status, it should not have affected distant recurrence rates negatively.
Survival analysis of the secondary groups divided by tumour characteristics showed that for patients with tumour size 11–20 mm, and for patients with receptor negative tumours and high S-phase fraction, there was a statistically significant decrease in distant recurrences. Tumour size and high S-phase fraction are both markers for high risk of distant recurrence, and in the programme of 1992 both of these variables were indicators for systemic therapy (a and b). With this taken into account, it seems probable that the extended use of systemic therapy based on the identification of high-risk groups in Period 2 influenced distant recurrence free survival rates. For approximately half of the patients information on S-phase fraction was missing due to the fact that tumour material sometimes was unavailable for analysis and that DNA analysis not always is informative as regards the SPF. Although most patients with missing information had small tumours (=10 mm), many other patients could have been recommended systemic therapy if the marker used had been informative. Therefore it might be important to evaluate proliferation markers with other techniques of high applicability, such as immunohistochemistry.
For patients with receptor positive tumours and low S-phase fraction there was a minor, statistically non-significant, increase in distant recurrence free survival. For this group of patients there was no change in recommendations of postoperative management between the periods, and neither did the use of systemic therapy increase significantly. However, from 1995 and onwards, the recommended duration of hormonal therapy was changed from two to five years, which means that this group of patients also had longer duration of treatment. In agreement with the extended recommendations of the management programme, patients with receptor positive tumours and high S-phase fraction received a higher frequency of hormonal therapy in Period 2. However, for this group of patients, there was no significant change in distant recurrence free survival. This was an unexpected result since it has been shown that patients with receptor positive tumours benefit from hormonal therapy Citation[15] and that distant recurrence free survival increases with longer duration of hormonal therapy Citation[16], Citation[26]. Neither has it been shown that high S-phase fraction should affect the beneficial effect of hormonal therapy negatively among receptor positive patients Citation[8], Citation[26]. No specific analysis of the relationship between distant recurrence free survival and duration of hormonal therapy was possible, due to lack of data.
The multivariate analysis showed that diagnosis period in itself had statistical significance for higher distant recurrence free survival, which implies that one or more factors not controlled for in the study were of importance for the prognosis. This result stayed true when the entire cohort was made analysable by replacing missing values for receptor status and SPF with corresponding mean values (data not shown). As mentioned above, we did not adjust for the duration of tamoxifen treatment and it is likely that the more frequent use of five years of treatment during Period 2 did contribute to the increase in recurrence-free survival. Since it has been shown that screened populations have higher survival rates Citation[27], Citation[28], one such possible factor is the screening schedule for breast cancer. Screening began in 1986, but it was not until the 1990s that there was a large screened population. Postoperative radiotherapy and systemic therapy have an additive effect for reducing distant recurrence risk Citation[29], Citation[30]. Since both of these regimens increased in Period 2, it might have influenced diagnosis period as a prognostic marker, although one should keep in mind that the effects of different regimens of radiotherapy used after mastectomy and breast conserving surgery, respectively, may not be equivalent. Yet another factor that might have affected the prognosis is the possible increase in the number of breast cancers linked to HRT during the studied time periods Citation[31].
Although data are not shown in results, there was a significant increase in distant recurrence free survival among patients not given systemic therapy in Period 2, compared to the corresponding group in Period 1 (36% lower recurrence risk, p = 0.027). The most likely reason for this is that during Period 2, the identification of high-risk and low-risk patients was more pronounced. When controlled for risk factors, the patients who did not receive any systemic adjuvant therapy had a higher frequency of tumours that were receptor positive, had a low S-phase fraction, and size ≤10 mm. On the other hand, the patients who did receive systemic therapy in Period 2 had as a group a higher frequency of high risk factors. This group had a slight tendency (p = 0.11) for lower recurrence risk in Period 2 compared to systemically treated patients in Period 1, despite the prognostic handicap. These results strengthen the hypothesis that the identification and selection of patients who would or would not benefit from systemic therapy was well grounded. It also illustrates that over-treatment would be a substantial problem if all the patients were given adjuvant chemotherapy. Given the ten-year distant recurrence-free survival rate in the first period of 85.4% () and a 30% risk reduction with adjuvant chemotherapy the estimated number needed to treat (NNT) would be over 20. On the other hand, for receptor negative patients with high S-phase tumours the NNT would be less than 10.
The multivariate analysis also showed that the S-phase fraction is a strong prognostic factor in stage I breast cancer. The use of gene-expression profiling to better identify patients at increased risk of recurrence has revealed that signatures related to cell proliferation might be the most important Citation[32–34]. We were not able to analyse tumour grade since data on grade was not continuously registered in the past. The NHG score is a simple and an important prognostic marker, however maybe not the optimal one, since tumours of intermediate grade can be separated into gene expression profiles representing a good or poor prognosis Citation[35].
When controlled, cases were discovered in which data was missing regarding cytotoxic therapy and distant recurrence, cases where cause of death was breast cancer but without any registration of distant recurrence, and cases with lack of follow-up because of patients who had moved from the region. This might illustrate some of the possible problems with studies based on historical databases, and highlights the probable need for at least some level of review of such data before analysis.
The survival rate of breast cancer is high among women diagnosed and treated in south-east Sweden. This accounts especially for stage I disease, for which the breast cancer specific ten-year survival rate for all patients diagnosed 1986 through 1999 was estimated to 90.9% Citation[5]. Our study has shown that the overall survival rate after ten years for patients < 60 years diagnosed 1986–1991 and 1992–1999 was 85.3% compared to 89.3% respectively, and the corresponding distant recurrence free survival rate was 85.4% compared to 90.0%. The conclusions are that the causes of the increase in overall survival and distant recurrence free survival for women with breast cancer stage I are complex. The continuous development of local and systemic treatment, together with changes in the regional management programme, has been beneficial. It is likely that women with high-risk stage I tumours have benefited from the extended systemic adjuvant therapy. Women with low-risk tumours seem to have a favourable prognosis even without systemic therapy. The continuing identification of risk groups and tailoring of treatment is of importance for overall survival and distant recurrence free survival for women with breast cancer stage I.
Acknowledgements
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Levi F, Lucchini F, Negri E, La Vecchia C. The decline in cancer mortality in the European Union, 1988–1996. Eur J Cancer 2000; 36: 1965–8
- Peto R, Boreham J, Clarke M, Davies C, Beral V. UK and USA breast cancer deaths down 25% in year 2000 at ages 20–69 years. Lancet 2000; 335: 1822
- Mouridsen HT, Bjerre KD, Christiansen P, Jensen M-B, Møller S. Improvement of prognosis in breast cancer in Denmark 1977–2006, based on the nationwide reporting to the DBCG Registry. Acta Oncol 2008; 47: 525–36
- Felay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol 2007; 18: 581–92
- Tejler, G, Norberg, B, Nordenskjöld B on behalf of the South East Sweden Breast Cancer Group. Survival after treatment for breast cancer in a geographically defined population. Br J Surg 2004;91:1307–12.
- Svenska bröstcancergruppen. Nationella riktlinjer för behandling av bröstcancer. http://www.swebcg.roc.se/natriktlinjbrca2007.htm.
- Stål O, Hatschek T, Carstensen J, Nordenskjöld B. DNA analysis in the management of breast cancer. Diagn Oncol 1991; 1: 140–54
- Stål O, Dufmats M, Hatschek T, Carstensen J, Klintenberg C, Rutqvist LE, et al. S-phase fraction is a prognostic factor in stage I breast carcinoma. J Clin Oncol 1993; 9: 1717–22
- Remvikos Y, Mosseri V, Asselain B, Fourquet A, Durand JC, Pouillart P, et al. S-phase fractions of breast cancer predict overall and post-relapse survival. Eur J Cancer 1997; 4: 581–6
- Daidone MG, Silvestrini R. Progostic and predictive value of proliferation indices in adjuvant therapy of breast cancer. J Natl Cancer Inst 2001; 30: 27–35
- Latinovic L, Heinze G, Birner P, Samonigg H, Hausmaninger H, Kubista E, et al. Prognostic relevance of three histological grading methods in breast cancer. Int J Oncol 2001; 6: 1271–7
- Rosen PP, Groschen S, Saigo PE, Kinne DW, Hellman S. A long term, follow-up study of survival in stage I (T1N0M0) and stage II (T1N1M0) breast carcinoma. J Clin Oncol 1989; 7: 355–66
- Sydöstra bröstcancergruppen. Vårdprogram för bröstcancer stadium I. Onkologiskt Centrum för Sydöstra Sjukvårdsregionen, Regionsjukhuset i Linköping, 1983.
- Sydöstsvenska bröstcancergruppen. Vårdprogram för bröstcancer. Sydöstsvenska Bröstcancergruppen och Onkologiskt Centrum för Sydöstra Sjukvårdsregionen, Universitetssjukhuset i Linköping, 1992.
- Early Breast Cancer Trialist's Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005;365:1687–717.
- Swedish Breast Cancer Cooperative Group (SBCCG). Randomized trial of two versus five years of adjuvant tamoxifen for postmenopausal early stage breast cancer. J Natl Cancer Inst 1996;88:1543–9.
- Ejlertsen B, Mouridsen HT, Jensen M, Andersen J, Cold S, Edlund P, et al. Improved outcome from substituting methotrexate with epirubicin: Results from a randomised comparison of CMF versus CEF in patients with primary breast cancer. Eur J Cancer 2007; 43: 877–84
- Fernö M, Stål O, Baldetorp B, Hatschek T, Källström A-C, Malmström P, et al. South Sweden Breast Cancer Group and South-East Sweden Breast Cancer Group. Results of two or five years of adjuvant tamoxifen correlated to steroid receptor and S-phase levels. Breast Cancer Res Treat 2000; 59: 69–76
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Ass 1958; 53: 457–81
- Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. Wiley, New York 1980
- Cox DR. Regression models and life tables (with discussion). J R Stat Soc B 1972; 34: 187–220
- Hammar M, Brynhildsen J, Dabrosin L, Frisk J, Lindgren R, Nedstrand E, et al. Hormonal replacement therapy and previous use of oral contraceptives among Swedish women. Maturitas 1996; 25: 193–9
- Brynhildsen J, Björs E, Skarsgård C, Hammar M. Is hormone replacement therapy a risk factor for low back pain among postmenopausal women?. SPINE 1998; 7: 809–13
- Ekblad S, Bergendahl A, Enler P, Ledin T, Möllen C, Hammar M. Disturbances in postural balance are common in postmenopausal women with vasomotor symptoms. Climacteric 2000; 3: 192–8
- Olsson HL, Ingvar C, Bladström A. Hormone replacement therapy containing progestins and given continuously increases breast carcinoma risk in Sweden. Cancer 2003; 6: 1387–92
- Fernö M, Baldetorp B, Bendahl PO, Borg A, Ewers SB, Olsson H, et al. Recurrence-free survival in breast cancer improved by adjuvant tamoxifen – especially for progesterone receptor positive tumours with a high proliferation. Breast Cancer Res Treat 1995; 36: 23–34
- Goldhirsch A, Colleoni M, Domenighetti G, Gelber RD. Systemic treatments for women with breast cancer: Outcome with relation to screening for the disease. Ann Oncol 2003; 14: 1212–4
- Jatoi I, Miller AB. Why is breast-cancer mortality declining?. Lancet Oncol 2003; 4: 251–4
- Overgaard M, Hansen PS, Overgaard J, Rose C, Andersson M, Bach F, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant adjuvant chemotherapy. NEJM 1997; 337: 949–55
- Overgaard M, Jensen MB, Overgaard J, Hansen PS, Rose C, Andersson M, et al. Postoperative radiotherapy in high-risk postmenopausal breast cancer patients given adjuvant tamoxifen. Lancet 1999; 353: 1641–8
- Schuetz F, Diel IJ, Pueschel M, von Holst T, Solomayer EF, Lange S, et al. Reduced incidence of distant metastastis and lower mortality in 1072 patients with breast cancer with a history of hormone replacement therapy. Am J Obstet Gynecol 2007; 196: 342e1–9
- Desmedt C, Sotiriou C. Proliferation: The most prominent predictor of clinical outcome in breast cancer. Cell Cycle 2006; 5: 2198–202
- Dai H, van't Veer L, Lamb J, He YD, Mao M, Fine BM, et al. A cell proliferation signature is a marker of extremely poor outcome in a subpopulation of breast cancer patients. Cancer Res 2005; 65: 4059–66
- Habel LA, Shak S, Jacobs MK, Capra A, Alexander C, Pho M, et al. A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients. Breast Cancer Res 2006; 8: R25
- Sotiriou C, Wirapati P, Loi S, Harris A, Fox S, Smeds J, et al. Gene expression profiling in breast cancer: Understanding the molecular basis of histologic grade to improve prognosis. J Natl Cancer Inst 2006; 98: 262–72