Abstract
Objectives. A prospective study of the efficacy and toxicity profile of patients with squamous cell carcinoma of the head and neck (HNSCC) without curative treatment options treated consistently with hypofractionated radiotherapy schedule. Patients and methods. Between 1995 and 2006, 158 patients with HNSCC, unsuitable for curative treatment, were treated with a hypofractionated scheme of radiotherapy consisting of 16 fractions of 3.125 Gy. Endpoints of the study were response rates, loco-regional control, disease-free survival, overall survival, acute and late toxicity, and quality of life (QoL). Results. Seventy four percent of patients were male, 31% had oropharyngeal cancer and 81% stage IV disease. With 45% complete response and 28% partial response an overall response rate of 73% was achieved, 6% had stable disease, and 21% progressed during or directly after completion of treatment. Median survival time was 17 months and 62 patients (40%) survived ≥1 year after RT. The actuarial rates of loco-regional control, disease-free survival and overall survival were 62%, 32% and 40% at 1-year, respectively and 32%, 14% and 17% at 3-years, respectively. Acute grade ≥3 skin and mucosal toxicities were observed in 45% and 65% of patients, respectively. Severe late toxicity was reported in 4.5% of patients. Of patients surviving ≥1 year after RT, retrospective chart review showed that 50% gained weight, pain improved in 77%, performance status in 47% and only 29% of them was still feeding-tube dependent. Conclusions. Our hypofractionated radiotherapy scheme is an effective, well-tolerated and safe palliative schedule in HNSCC who are unsuitable for curative treatment options. Using 3.125 Gy per fraction (Christie scheme), excellent palliation was achieved resulting in acceptable response rates, excellent symptom control, acceptable toxicity profile, and good QoL of patients surviving ≥1 year after completion of treatment.
A substantial number of patients with squamous cell carcinoma of the head and neck (HNSCC) are unsuitable for aggressive radical treatment with surgery or chemoradiotherapy (CRT) because of a very advanced loco-regional disease, significant co-morbidities, poor performance status, distant metastatic disease, or a combination of these factors. However, this group of patients still requires some form of treatment to control their loco-regional disease and to alleviate their troublesome symptoms. Considerations for an optimal palliative radiotherapy (RT) schedule are: significant tumor regression and symptom control within a short overall treatment time (OTT) with minimal side effects. Frequently some form of hypofractionation is opted for. The benefit of an increased tumor cell kill because of the large fraction size in a short OTT is counteracted, from radiobiological point of view, by an increased potential for late side effects Citation[1]. However, late radiation toxicity is often less relevant in patients treated in palliative setting.
The Christie Hospital in Manchester developed a 3-week schedule of RT during World War II when RT facilities were limited. Results were found not to be different from the conventional schedules used in the previous treatment periods in terms of local control and toxicity Citation[2], Citation[3]. This schedule was, therefore, adopted by Christie hospital and number of other British cancer centers as a standard RT schedule for early-stage laryngeal cancer. Many randomized and non-controlled trials have also shown no difference in local control between conventional and hypofractionated schedules Citation[3–7]. Surprisingly, many of these schedules gave less severe late normal tissue reaction than expected given the short OTT and the high fraction dose Citation[3–6], Citation[8], Citation[9]. Because of these encouraging outcomes, the Erasmus MC-Daniel den Hoed Cancer Center adopted for a cohort of palliative patients a hypofractionated radiation schedule, comparable to that used in the Christie hospital.
The purpose of this study is twofold. First, to evaluate the response rates, toxicity and survival in the patients treated according to the “Christie scheme”. Second, to determine the compliance and the impact of this schedule on quality of life (QoL) in patients surviving ≥1 year after completion of treatment.
Patients and methods
Between 1995 and 2006, 158 patients with biopsy-proven HNSCC were considered unsuitable for definitive treatment with surgery or CRT and offered, therefore, a hypofractionated schedule of RT at the Erasmus MC-Daniel den Hoed Cancer Center. The reason for these patients considered to be unsuitable for curative treatment options were: major comorbidities and poor performance status (n = 25), massive tumor (n = 23), distant metastases (DM) (n = 15), second or third primary cancer (n = 11), synchronous tumor outside the head and neck region (n = 10), synchronous head and neck cancer (n = 5), high age > 90 year (n = 4), or the combination of one or more of the aforementioned factors (n = 61). The reason was not mentioned in the remaining four patients (). The treatment was, therefore, given with a palliative intention in order to achieve a maximal durable loco-regional and symptom control. All patients were retrospectively staged according to the standards of the 2004 TNM-staging system.
Table I. Patients, tumor, and treatment characteristics.
Radiotherapy
Patients were immobilized in a supine treatment position in a custom-made head-and-neck mask manufactured in the mould room. All patients underwent simulation, using conventional or CT planning. The radiation field encompasses the gross disease (primary tumor and/or nodal disease) with a 1 cm margin. Two lateral fields were mostly used to treat the primary tumor and/or upper neck with a matched anterior field, as needed for the supraclavicular region. The intended radiation dose was 50 Gy in 16 fractions of 3.125 Gy, given 5 times a week (biologically equivalent to 54.7 Gy in 2 Gy fractions using an α/β ratio of 10) Citation[1]. After 12 fractions, the spinal cord was shielded and electrons (usually 9–10 MeV) were used to treat the posterior triangles in the last 4 fractions. The maximal spinal cord dose was 37.5 Gy (biologically equivalent to 48 Gy in 2 Gy fractions using an α/β ratio of 2) Citation[1]. Surface bolus was used for nodal disease with skin invasion or fungation. Chemotherapy (CT) was allowed and given to 16 patients with massive tumors as induction therapy. In case of poor medical condition, the attending radiation oncologist elected for 4 fractions of 3.125 Gy per week.
The RT was given 5 times a week in 81 patients (51%) and was completed within a median time of 22.6 (range: 22–24) days. Seventy-seven patients (49%) were irradiated 4 times a week and completed treatment within a median time of 26.3 (range 25–30) days. Sixteen patients received induction chemotherapy because of massive primary and/or nodal disease; 2 to 5 courses of cisplatin and 5-fluorouracil or cisplatin were given. Because of the poor response to chemotherapy, these patients were considered incurable and offered this hypofractionated scheme of RT in a palliative setting.
Endpoints
End points of the study were response rates (complete response [CR], partial response [PR], and overall response rate [ORR]) (ORR = CR + PR), loco-regional control (LRC), disease-free survival (DFS), overall survival (OS), acute and late toxicity and retrospective assessment of QoL. The treatment response was evaluated by the head and neck surgeon or radiation oncologist 6–8 weeks after completion of RT and was done by clinical examination and by CT or MRI of the head and neck. Given the potential for differential response at primary and nodal sites in the same patient, the treatment response was recorded as CR only when both primary and nodal disease disappeared completely. If there was any residual disease (either locally or regionally), the case was recorded as PR. Patients in whom the primary tumor and/or nodal disease did not respond to RT were recorded as stable disease (SD), while patients who progressed during or directly after the treatment were recorded as progressive disease (PD). LRC were reported. Local and/or regional failures were recorded as events. Patients died from intercurrent disease without evidence of loco-regional failure were censored at the moment of death. DFS was measured from the date of completion of treatment to the date of first relapse (loco-regional or distant metastases) or death. Acute and late toxicities were evaluated by the radiation oncologist during each visit of patients to the outpatient clinic of our hospital according to the RTOG/EORTC acute and chronic radiation morbidity scoring criteria Citation[10]. All patients were encouraged to maintain oral food intake and in case of difficulty, feeding tube was inserted either through the nasal route, percutaneously, or endoscopically. For patients with respiratory distress, it was sometimes elected to perform tracheostomy before starting RT.
QoL assessment was done retrospectively in patients who were still alive with no evidence of disease (NED) at the time of this analysis (n = 12) by using the EORTC QLQ-C30 (version 3.0) and EORTC H&N35 QoL-questionnaires Citation[11]. Subjective response as an indirect measure of QoL was also assessed retrospectively using chart review in patients who survived beyond 12 months (n = 62) by recording the evolution in time of different relevant items of QoL such as pain control, improvement in performance status, ability to eat liquid or solid food again, and weight gain. The scores were graded as improved, worsened or not changed.
Follow-up
Following completion of treatment, patients were followed up 2-monthly for the first year, 3-monthly for the second and third year and 6-monthly thereafter. At each visit, medical history and routine clinical ENT-examination were performed, including flexible nasoendoscopy, when indicated.
Statistical analysis
Survival rates were calculated from the completion of treatment using Kaplan-Meier technique. Possible predictive clinical factors for ORR, DFS and OS (tumor site, tumor stage, age, sex, use of chemotherapy, RT-schedule, performance status, and co-morbidity), were tested using the χ2 test. All significance tests were two-sided and statistical significance was accepted for a calculated p-value of < 0.05.
Results
Baseline characteristics were listed in . Median age was 68.5 (range: 41–95) years; 74% of patients were males, 22% were 80 years and older and 60% had a significant co-morbidity. Median follow-up time was 16.4 months (range: 1–122) with 20% of patients having a follow-up time longer than 2 years. and show the distribution of patients by site and stage and by TNM-classification, respectively.
Table II. Disease by site and stage.
Table III. TNM staging of tumor.
Objective response to radiotherapy
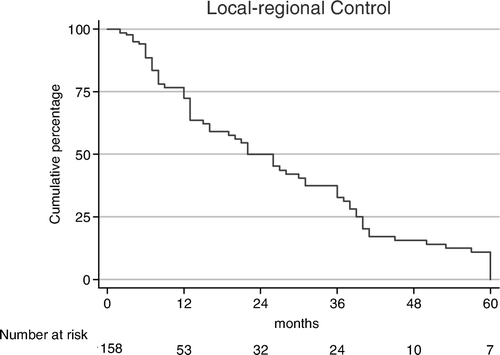
Seventy-one patients (45%) had a CR and 44 (28%) a PR, resulting in an ORR of 73%. Nine patients (6%) had SD and 34 (21%) had PD during or directly after the RT and died shortly thereafter. ORRs were significantly related to tumor stage and tumor site. As shown in , ORRs for stage I/II, stage III/IVA/IVB and IVC were 94%, 71% and 60%, respectively (p = 0.02). For cancers of larynx, oropharynx, oral cavity, and hypopharynx ORRs of 91%, 75%, 68%, and 61%, respectively (p = 0.04), were found. From 16 patients who received induction chemotherapy because of bulky tumors, seven patients (44%) had a CR after completion of RT, four (25%) had PR for an ORR of 69%, compared to 73% of the whole group. The remaining five patients who received induction CT had either SD or PD. As shown in , the actuarial incidences of LRC were 62%, 32%, and 12% at 1, 3, and 5 years, respectively.
Table IV. Overall response rates, disease-free survival and overall survival at 1-year.
Disease-free survival
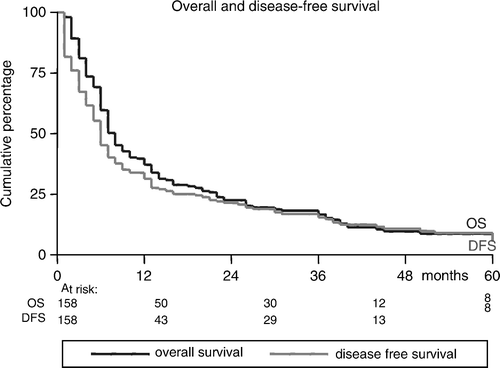
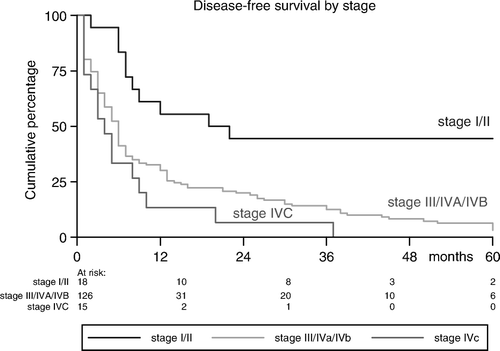
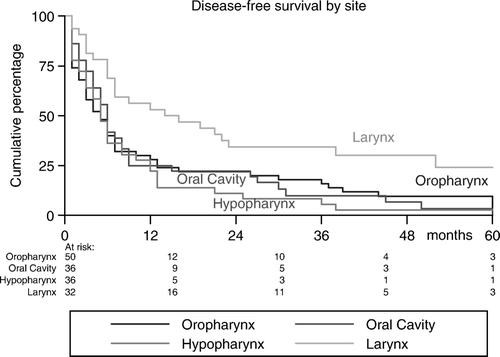
Sixteen of the 71 complete responders (22%) progressed with a median DFS of 14 (range: 3–38) months; eight patients progressed loco-regionally, seven patients developed DMs and one patient developed both local recurrence and DM. Partial responders progressed after a shorter DFS (5.5 months, range: 1–13), while patients with SD progressed after a median DFS of 2.5 (range: 2–5) months. The actuarial incidences of DFS were 32%, 14%, and 5% at 1, 3, and 5 years, respectively (). As shown in , and , DFS-rates were tumor stage- and site-related (p = 0.005 and p = 0.02, respectively).
Overall survival
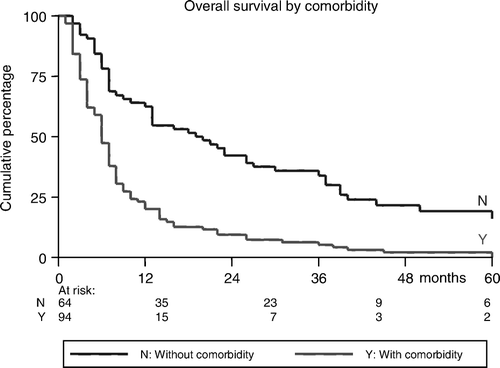
Median survival of the whole group is 17 (range: 1–151) months. Sixty-two patients (40%) survived ≥1 year. The actuarial incidences of OS were 40%, 17%, and 5% at 1, 3, and 5 years, respectively (). Median survival and OS were significantly better in patients without co-morbidities than in patients with major co-morbidities (28.1 months and 65% vs. 10.1 months and 23%, respectively, p < 0.0001) (). At the time of analysis, 12 patients were still alive NED. From the 146 deaths, 98 deaths (67%) were HNSCC-related and 48 deaths (33%) were non-HNSCC-related; 38 patients died of intercurrent disease and ten died of a new primary cancer. The average time to death from HNSCC was 8.8 (range: 1–40) months and from HNSCC-unrelated reason 21.5 (range: 1–129) months. As shown in , OS was shorter in patients with higher tumor stage (p = 0.04) and in patients with cancers of the hypopharynx and oral cavity than in cancers of the larynx and oropharynx (p = 0.08). Age, sex, performance status, use of chemotherapy, and the used RT-schedule (4 vs. 5 times a week), were all found to have no significant association with ORR, DFS, and OS.
Subjective response to treatment
Because prospective QoL-assessment was not done in our patients, subjective response was assessed retrospectively in patients who survived ≥12 months after completion of treatment (n = 62; 40%). Chart review included pain control, improvement in performance status, ability to eat (semi)solid food and weight gain. As shown in , pain was improved in ten of 13 patients using opiate analgesics (77%) and the performance status in 14 of 30 patients who had WHO-performance status ≤ 2 before RT (47%). Fifty-percent of patients with reported weight loss before starting treatment had gained weight and 24% had stable weight. Thirty-one of 48 patients (65%) who were feeding-tube dependent were able to eat (semi)solid food again. In 14 patients (29%) the feeding status was not improved and they were still feeding-tube dependent one year after RT.
Table V. Chart review of patients survived ≥1 year after completing the treatment.
Acute toxicity
None of the patients died because of acute radiation toxicity. Grade 3 moist skin desquamation and confluent mucositis were reported in 71 (45%) and 102 (65%) patients, respectively. Despite the high rate of acute skin and mucosal toxicities, there was no treatment break due to acute side effects of treatment. This was considered to be an indirect measure of tolerability of treatment in these patients. Forty-eight patients (30%) had severe pain which required opiate analgesics. A feeding tube was used in 103 patients (65%): 32 (20%) had feeding tube sited before starting treatment and 71 patients (45%) experienced acute grade ≥3 dysphagia during the course of RT and required insertion of a feeding tube.
Late toxicity
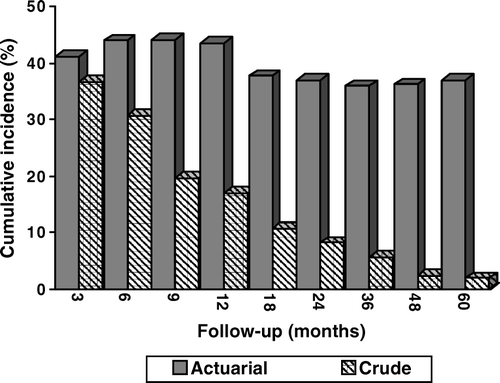
Almost all patients had some complaints related to the RT. As shown in , the actuarial incidences of grade ≥2 late RTOG/EORTC toxicity were 43% and 37% at 1- and 2-years, respectively, while the crude incidences of grade ≥2 late toxicity were 17% and 8%, respectively at 1- and 2-years. Late feeding problems, defined as feeding-tube dependency was seen in 29% of the patients who survived ≥12 months after completion of RT. Severe late grade 4 toxicity was reported in seven patients (4.5%), only one of them survived 1 year beyond the RT (13 months) and had an oesophageal stricture requiring dilatation. Two patients with osteoradionecrosis of the mandible, four patients with an oesophageal stricture and one with a persisting oropharyngeal ulcer died within 12 months after completion of the treatment.
QoL assessment
QoL was assessed retrospectively in patients who were still alive NED at the time of this analysis (n = 12) by using the EORTC QLQ-C30 (version 3.0) and EORTC H&N35 QoL-questionnaires. Of those patients, nine completed and returned the questionnaires (response rate 75%). The median age of this group was 66.5 years and the median survival was 26 months. Half of them had early-stage disease and the other half a loco-regional advanced disease. Mean global health status was calculated to be 71. In this group of patients, the highest symptom scores of EORTC QLQ-C30 were dyspnoea, insomnia and constipation. Among functional scales; physical functioning was the worst and role functioning ranked the highest scores. In the H&N-35 module, dry mouth and sticky saliva were ranked amongst the highest scores, followed by swallowing problems.
Discussion
Patients with untreated advanced stage HNSCC have a median survival of approximately 100 days Citation[12]. Historically, patients with unresectable HHSCC treated by RT alone have LRC rates between 50 and 70% Citation[13–15] and 5-year survival rates of 10–20% Citation[13], Citation[15]. Most of these patients die of loco-regional disease progression. In a curative setting, the addition of CT in different regimens has improved disease control and long-term survival Citation[13], Citation[14]. However, patients deemed inoperable and not fit to withstand the burden of CRT, as is the case in our study population, still require some form of palliative treatment to control their loco-regional disease and to alleviate their disturbing symptoms. Although, the information about the optimal palliative regimen for incurable HHSCC in the current literature is scanty, an optimal palliative RT schedule is one that would provide worthwhile regression of the tumor and local symptoms within a short OTT with minimal toxicity. From radiobiological, economic and logistical points of view, a hypofractionated schedule would be the most suitable option. First, the treatment is completed before accelerated repopulation becomes a significant radiobiologic factor. Second, the reduction in the number of fractions also allows a more efficient use of resources, which can help avoid long waiting times for other patients and lastly, considering that this group of patients are usually of age and often have a poor performance status as well as significant co-morbidities, it is almost mandatory to keep the OTT as short as possible. Because of the aforementioned advantages of a hypofractionated treatment schedule, we treated this group of HNSCC unsuitable for curative treatment options with 16 fractions of 3.125 Gy, comparable to the scheme developed at the Christie Hospital in Manchester for glottic cancer in curative setting. We have achieved an excellent response rates (CR 45% and ORR = 73%), reasonable LRC-rates (62% and 32% at 1- and 3-years, respectively), reasonable DFS-rates (32% and 14% at 1- and 3-years, respectively), and reasonable OS-rates (40% and 17%, at 1- and 3-years, respectively), with high rates of acute toxicities and acceptable late toxicity profile in this frail group of patients. At the time of diagnosis, all our patients upfront had a very poor outlook based on their massive tumor, bad general condition, sever co-morbidities and/or age. Baatenburg de Jong et al. Citation[16] have developed a prognostic model to predict survival in patients with HNSCC, which may be useful in patient counseling and in clinical decision-making. When we applied this model to our patient population retrospectively, we obtained expected median 1- and 5-year survival rates of 13% and 0.6%, respectively, purely based on their pre-treatment characteristics. These figures indicate the dismal prognosis per se of this patient subset. The results of LRC in our study are quite comparable with those reported in different randomized and non-controlled clinical investigations using hypofractionated schedules of RT Citation[3–7], Citation[13], Citation[15], Citation[17], in fairly comparable patient populations. The compliance was good as there was no treatment break despite the high rate of acute grade 3 skin and mucosal side effects. Severe late complications, defined as grade 4 toxicity arising more than 6 months after RT, occurred in seven cases in our series (4.5%), which is comparable with published results of other studies with hypofractionated schedules Citation[3–6] and across the general population of HNSCC Citation[17]. However, longer follow-up might have resulted in higher rates of late toxicity, but evaluation of late toxicity remains difficult in this group, since only 5% of patients survive beyond 5 years.
The good local control and the relatively higher (but probably acceptable) toxicity profile achieved in our patients were not totally surprising. In terms of radiobiological modelling, 50 Gy in 16 fractions, 5 times a week with a median treatment time of 23 days, compares favourably with conventional once daily fractionation of 70 Gy in 35 fractions with 7 weeks (47 days), taking into account the high fraction dose (biologically equivalent to 54.68 Gy in 2 Gy fractions using an α/β ratio of 10) and the correction for the short OTT using an average Dprolif of 0.64 for head and neck cancers Citation[1]. Another possible explanation of the results of outcome and toxicity in our study is the fact that the reduction in total dose used in our patients is sufficient to compensate for any possible adverse effect of increased fraction size, while the shorter OTT maintains tumor control Citation[1]. Lastly, the good local control in our patients might also be due to the inclusion of 14 patients with early-stage glottic cancers (stages I/II with very bad condition and/or age > 90 year) with an ORR of 93%, compared to 73% ORR of the whole group. From a radiobiological point of view, HNSCC is generally known to have low fractionation sensitivity, a high α/β ratio and therefore small fraction sizes are advised. Most of early-stage glottic cancers are well- or moderately-differentiated, slowly growing, have low α/β ratio and therefore sensitive to high fraction size Citation[1]. This would explain the high ORR achieved in those 14 patients with early-stage glottic cancer. Mendenhall et al. Citation[18] reported 100% local control rate in T1 glottic cancers treated with 2.25 Gy, compared to 80% in those treated with fraction size of 2 Gy.
Our results were similar to the findings in the study of Levendag et al. Citation[15] and Schofield et al. Citation[3] who suggested that LRC and DFS rates are better in tumors of the oropharynx and larynx, compared to those of oral cavity and hypopharynx. For all tumor sites, early-stages fare better than locally advanced disease (III/IVA/IVB). Comorbidity was significantly associated with lower DFS and OS-rates in our patients. Sixteen patients with massive tumors who received induction CT with poor response were considered incurable and were offered this scheme of hypofractionated RT. They also responded insufficiently to RT with an ORR of 69%, compared to 73% of the whole group. Ensley et al. Citation[19] showed that 97.8% of the initial responders to CT also responded to RT, while only 5.5% of initial no-responders subsequently responded to radiotherapy, suggesting that HHSCC sensitive to initial CT shares parameters that are also radiation sensitive. Other patients demographics as age, sex, performance status, and the used RT-schedule (4 vs. 5 times a week), were not found to be predictors for ORR, DFS and OS of significance.
Beside the results of LRC, survival, and toxicity, the impact of any palliative schedule on QoL is an important issue to be addressed by evaluating such treatment schedule. Therefore, we have assessed QoL retrospectively in patients who were still alive at the time of this analysis with NED by using the EORTC QLQ-C30 (version 3.0) and EORTC H&N-35 QoL-questionnaires. This group of patients had a mean global health score of 71. These results are comparable with those reported in studies were specific elements of the EORTC QOL-C30 were used. Corry et al. Citation[5] reported an improvement in QoL in 44% of patients after RT for incurable HHSCC. In patients who surviving ≥1 year (n = 62), the subjective response was evaluated retrospectively as indirect measurement of the impact of such treatment regimen on different items of QoL, using chart review. In our opinion, this hypofractionated schedule impacts positively on QoL of those patients. Pain improved in 77% and performance status in 47% of those patients. Only 29% of patients who survived beyond 1 year after completion of treatment was still feeding-tube dependent, while 65% of them was able to eat (semi)solid food again. Fifty-percent of patients survived ≥1 year also gained weight.
The limitations of the current study, including the biases inherent to a retrospective review, are well understood by the authors. It is also acknowledged that there was selection bias in the present study due to inclusion of patients with early-stage laryngeal cancer deemed unsuitable for curative options because of high age, poor performance and/or co-morbidity. This inclusion bias would partially explain the excellent LRC-rates. However, most of these patients died of intercurrent disease which explains the relatively lower OS rates. Furthermore, the assessment of QoL was done retrospectively on the basis of chart review in patients survived ≥12 months. However, QoL was also assessed in patients who were still alive with NED at the time of this analysis (n = 12), using the EORTC H&N35 QoL-questionnaires despite the absence of baseline QoL assessment. Lastly, only 5% of our patients survived ≥5 years. This makes it difficult to draw general conclusions regarding the late radiation-induced morbidities. Within these given limitations of the study, however, the current study provides valuable data on the efficacy and safety of this hypofractionated schedule in a palliative setting for HNSCC patients who are unsuitable for curative options. In our opinion, the observations mentioned in this paper have also important implications for clinicians faced with the problem of choosing the most convenient and effective palliative schedule in such group of patients.
Conclusions
At the time of diagnosis, all our patients had a dismal prognosis and thus a short life-expectancy on the basis of their demographic features, but required some kind of palliative treatment. Despite this pessimistic starting-point of the study, excellent local and symptom control was achieved in the majority of patients. Forty percent have survived beyond 1 year after the treatment and about two-third of these patients showed improvement in different items of QoL. Because of these encouraging results and its radiobiological, economic and logistical advantages, this hypofractionated scheme has been adopted by our institution as the standard palliative RT-scheme for patients with HNSCC who are unsuitable for curative treatment options as surgery or CRT.
Acknowledgements
We want to thank J. de Vreugd for her effort in collecting patient's data.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Steel, GG. Basic clinical radiobiology. 3rd ed. New York: Hodder Arnold; 2002.
- Paterson R. Studies in optimum dosage. Br J Radiol 1952; 25: 505–16
- Schofield CP, Sykes AJ, Slevin NJ, Rashid NZZ. Radiotherapy for head and neck cancer in elderly patients. Radiother Oncol 2003; 69: 37–42
- Weissberg JB, Son YH, Percarpio B, Fischer JJ. Randomized trial of conventional versus high fractional dose radiation therapy in the treatment of advanced head and neck cancer. Int J Radiat Oncol Biol Phys 1982; 8: 179–85
- Corry JF, Peters LJ, D'Costa I, Milner AD, Fawns H, Rischin D, et al. The “Quad Shot”– a phase II study of palliative radiotherapy for incurable head and neck cancer. Radiother Oncol 2006; 77: 137–42
- Henk JM, James KW. A comparative trial of large and small fractions. Br J Radiol 1976; 49: 1055–6
- Porceddu SV, Rosser B, Burmeister BH, Jones M, Hickey B, Baumann K, et al. Hypofractionated radiotherapy for the palliation of advanced head and neck unsuitable for curative treatment – “Hypo Trial”. Radiother Oncol 2007; 85: 456–62
- Wierink G, Alcock CJ, Bated TD, Brindle JM, Fowler JF, Gajek WR, et al. Final report on the second British Institute of Radiology fractionation study: Short versus long overall treatment times for radiotherapy of carcinoma of the laryngopharynx. Br J Radiol 1991; 64: 232
- Gowda RV, Henk JM, Mais KL, Sykes AJ, Swindell R, Slevin NJ. Three weeks radiotherapy for T1 glottic cancer: The Christie and Royal Marsden Hospital experience. Radiother Oncol 2003; 68: 105–11
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and European Organisation for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995; 31: 1341–6
- Bjordal, K, de Graeff, A, Fayers, PM, Hammerlid, E, van Pottelsberghe, Curran, D, , et al. A 12 country field study of the EORTC QLQ-C30 (version 3.0) and the head and neck cancer specific module (EORTC QLQ-H&N35) in head and neck patients. EORTC Quality of Life Group. Eur J Cancer 2000; 36:1796–807.
- Kowalski LP, Carvalho AL. Natural history of untreated head and neck cancer. Eur J Cancer 2000; 36: 1032–7
- Laskar SG, Agarwal JP, Srinivas C, Dinshaw KA. Radiotherapeutic management of locally advanced head and neck cancer. Expert Rev Anticancer Ther 2006; 6: 405–17
- Bonner JA, Giralt J, Harari PM, Ang KK, Cohen RB, Kies MS, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Eng J Med 2006; 354: 567–78
- Levendag PC, Nowak PJ, van der Sangen MJ, Jansen PP, Eijkenboom WM, Planting AS, et al. Local tumor control in radiation therapy of cancers in the head and neck. Am J Clin Oncol 1996; 19: 469–77
- Baatenburg de Jong RJ, Hermans J, Molenaar J, Briaire JJ, le Cessie S. Prediction of survival in patients with head and neck cancer. Head Neck 2001; 9: 718–24
- Mendenhall WM, Parsons JT, Mancuso AA, Stringer SP, Cassisi NP. Radiotherapy for squamous cell carcinoma supraglottic larynx: An alternative to surgery. Head Neck 1996; 18: 24–35
- Mendenhall WM, Parson JT, Million RR, Fletcher GH. T1-T2 squamous cell carcinoma of the glottic larynx treated with radiation: Relationship of dose-fractionation factors for local control and complications. Int J Radiat Oncol Biol Phys 1988; 15: 1267–73
- Ensley JF, Jacobs JR, Weaver A, Kinzie J, Crissman J, Kish JA. Correlation between response to cisplatinum-combination chemotherapy and subsequent radiotherapy in previously untreated patients with advanced squamous cell cancers of the head and neck. Cancer 1984; 54: 811–4