To the Editor
The recent introduction of molecularly targeted anticancer agents has dramatically improved outcomes for patients with metastatic renal cell carcinoma (RCC). One such agent is sunitinib malate (SUTENT®, Pfizer Inc), an oral, multitargeted receptor tyrosine kinase inhibitor that is approved multinationally for the treatment of advanced RCC and imatinib-resistant/-intolerant gastrointestinal stromal tumour (GIST), and has been shown to significantly extend progression-free and overall survival in these settings Citation1–3.
As targeted agents become more widely prescribed as a result of the clinical benefit in mRCC patients, a clearer picture of their potential toxicities is emerging. Cardiotoxicity with these agents has been reported in clinical trials and in clinical practice Citation4–6, although the incidence, the potential pathological mechanisms at work, and the risk factors for heart failure in these patients are the subject of ongoing debate. Schmidinger et al. demonstrated that cardiac events (defined as the occurrence of increased enzymes if normal at baseline, symptomatic arrhythmia that required treatment, new left ventricular dysfunction or acute coronary syndrome) associated with target therapies are manageable with careful cardiovascular monitoring and early treatment Citation[4]. It is clear that good cooperation between cardiologists and oncologists is essential to improve prognosis and survival in patients with heart disease and coexisting neoplastic diseases.
We report here the successful management of a case of significant reversible heart failure in a young woman with mRCC treated with sunitinib. We believe this is the first reported case that demonstrates that, in patients with mRCC who experience heart failure during treatment with sunitinib, effective management of the symptoms can allow patients to continue sunitinib treatment and achieve optimal benefit from the treatment.
Case report
A 32-year-old woman with RCC and metastases in the intrathoracic and intra-abdominal lymph nodes had right-sided nephrectomy. Carcinoma clarocellulare was diagnosed following histopathological analysis. There was no family history of heart disease and no other risk factors for cardiovascular disease. The patient was in good physical health and was considered suitable for treatment with sunitinib. Sunitinib was initiated at 50 mg/day on 6-week cycles of 4 weeks on treatment followed by 2 weeks off treatment (Schedule 4/2). After two cycles of sunitinib, the patient presented with hypertension. Her blood pressure was 160/100 mmHg, compared with 120/80 mmHg at baseline. Amlodipine 5 mg/day was prescribed for her hypertension and she continued sunitinib. Blood pressure decreased to ≤140/90 mmHg; tumour stabilisation was observed.
After 1 year of sunitinib (during cycle 9), the patient experienced breathlessness induced by mild exercise. The attacks were self-limiting, lasted only a few minutes, and were not associated with chest pain, palpitations or syncope. On examination, her pulse was 110 beats/minute and regular, and blood pressure was 100/60 mmHg. Auscultation revealed crackles at the base of both lungs. Peripheral oedema was also reported. The electrocardiogram (ECG) revealed tachycardia with sinus rhythm and no abnormalities in comparison with the baseline ECG. A chest x-ray showed mild passive congestion in her lungs. A control radioisotope MUGA (MUltiple Gated Acquisition) scan revealed substantially reduced left ventricular ejection fraction (LVEF), with a considerable increase in end-diastolic and end-systolic diameter of the left ventricle and changes in diastolic function (, and ). Biochemical tests showed no signs of cardiac necrosis serum marker elevation (troponin was <0.03 ng/ml [normal value: 0–0.4 ng/ml]), and no liver or kidney dysfunction; haemoglobin levels were normal (127 g/L [116–162 g/l]). Thyroid function tests were not performed and testing for brain natiuretic peptide (BNP) was not possible.
Figure 1. MUltiple Gated Acquisition (MUGA) scan showing left ventricular ejection fraction (LVEF), end-diastolic (ED) diameter and end-systolic (ES) diameter at baseline.
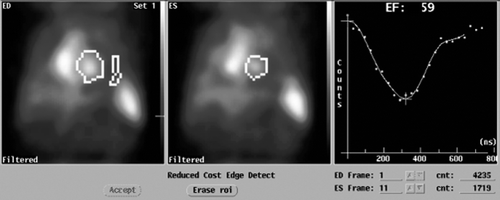
Figure 2. MUltiple Gated Acquisition (MUGA) scan showing left ventricular ejection fraction (LVEF), end-diastolic (ED) diameter and end-systolic (ES) diameter 1 year after the start of sunitinib treatment when cardiac symptoms appeared.
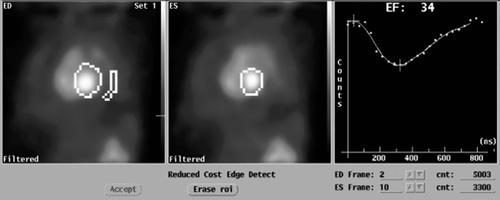
Table I. Results of four tests of MUltiple Gated Acquisition (MUGA) blood pool in a patient with symptoms of heart failure after treatment with sunitinib.
On the recommendation of a cardiologist, cardiac symptoms were treated with a diuretic (furosemide, 80 mg/day) and a low-dose beta-blocker (metoprolol 25 mg/day). Cardiac treatment was given during the 2-week off-treatment period of cycle 9 to ensure uninterrupted treatment with sunitinib. After cardiac recompensation, the patient continued treatment with sunitinib at a lower dose of 37.5 mg/day on Schedule 4/2 (75% of the previous dosage).
Cardiac function improved after 6 months and normalised after 8 months. MUGA scans revealed normal systolic myocardial function (ejection fraction), improved diastolic function and normal end-diastolic and end-systolic diameter of the left ventricle (, and ). Heart failure was reversed. A computed tomography (CT) scan showed stable RCC with no change in the size of metastases.
Figure 3. MUltiple Gated Acquisition (MUGA) scan showing left ventricular ejection fraction (LVEF), end-diastolic (ED) diameter and end-systolic (ES) diameter 6 months after the start of therapy for cardiac symptoms.
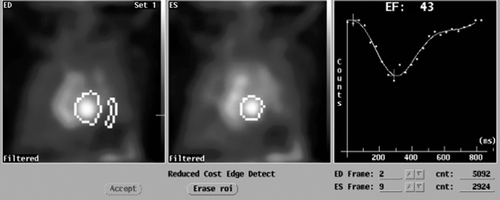
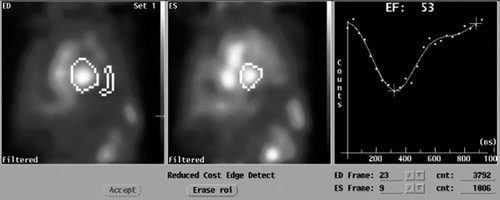
Figure 4. MUltiple Gated Acquisition (MUGA) scan showing left ventricular ejection fraction (LVEF), end-diastolic (ED) diameter and end-systolic (ES) diameter 8 months after the start of therapy for cardiac symptoms.
However, 4 months after the incidence of heart failure, hypothyroidism was detected: thyroid stimulating hormone (TSH) was 227 µIU/mL (0.4–4.0 µIU/mL) and free thyroxine (T4) was 0.160 ng/dL (0.93–1.7 ng/dL). Hypothyroidism was adequately managed with thyroxine supplementation.
Discussion
This case documents significant reversible myocardial dysfunction and heart failure in a patient without cardiovascular risk factors treated with sunitinib for mRCC. The first cardiovascular complication was hypertension, which was controlled with the calcium channel blocker, amlodipine. The choice of amlodipine was dictated by the patient having only one kidney. However, published research suggests an angiotensin-converting-enzyme inhibitor may have been the best choice Citation[7].
After 1 year of sunitinib treatment, the patient experienced heart failure symptoms. A MUGA scan confirmed cardiac dysfunction, and subsequent improvement of heart function after therapy with furosemide and metoprolol. In spite of being more invasive compared with transthoracic echocardiography, the MUGA scan is free from investigator bias and measurements obtained are therefore more accurate and reproducible than those obtained with other techniques.
Significantly, the typical markers for cardiac damage (troponin, creatine kinase-MB) were not elevated and there were no ECG changes with the exception of sinus tachycardia. Despite the presence of reversible cardiac dysfunction, no permanent necrotic damage in the cardiomyocytes or changes in cardiac conductivity were noted. There was co-existing hypothyroidism.
After successful treatment of oedema, sunitinib was continued at a lower dose. With concomitant treatment for cardiac symptoms for a couple of months, heart muscle function and heart chamber sizes returned to normal. The cancer remained stable throughout treatment.
Published evidence shows sunitinib to be an effective treatment for mRCC Citation[1] and advanced GIST Citation[3], but reports vary with respect to the incidence of cardiotoxicity Citation[5]. In a clinical trial with sunitinib in patients with advanced GIST, 2% of patients had ≥20% decreases in LVEF, and below the lower limit of normal, while 0.7% of patients had treatment-related' cardiac failure', 'cardiac failure congestive' or 'left ventricular failure' Citation[8]. In an analysis of a phase I/II trial in patients with GIST, 11% of patients receiving sunitinib had cardiovascular complications, 8% had symptoms of heart failure and 28% had decreased LVEF of 10%. A 15% LVEF reduction was seen in 19% of patients and 47% of patients experienced hypertension (>150/100 mmHg) Citation[6]. Using animal models, sunitinib was found to cause injury to mitochondria and apoptosis of cardiomyocytes Citation[6]. In previously untreated patients with mRCC, 10% of patients receiving sunitinib showed a decrease in LVEF, but there were no significant cardiovascular adverse events after median 6 months of treatment Citation[1]. In an analysis of short- and long-term safety in a sunitinib expanded-access study in patients with mRCC (N = 4 622), 1% of patients had cardiac disorders Citation[9].
The possible pathomechanism of cardiotoxicity during therapy with sunitinib, and the potential risk factors for heart failure, are the subject of debate. Sunitinib may block metabolic pathways that are indispensable for cardiomyocytes to function normally. However, not all patients treated with sunitinib develop cardiotoxicity, suggesting potential genetic predisposition. In addition, there is evidence that sunitinib damages the endothelium Citation[10]. It has also been suggested that the co-existence of hypothyroidism and heart failure during the sunitinib therapy may be significant Citation[5].
This case shows that heart failure during sunitinib therapy may appear in young people without cardiovascular risk factors, is reversible, and may co-exist with hypothyroidism. Patients should be monitored for hypertension and symptoms of cardiac dysfunction Citation[11]. Exertional dyspnoea and fluid overload may be an indication of heart failure and the symptoms can be reduced with the use of diuretics and beta-blockers. Hypertension may play a role in causing injury to the heart and should be stabilised. Sunitinib dose reduction prevents the return of heart failure and allows the continued, effective treatment of mRCC. Collaboration between the cardiologist and the oncologist in this case allowed the patient to continue sunitinib treatment. The patient has continued to experience stable disease despite the dose reduction.
To our knowledge, this is the first reported case showing that in patients with mRCC who experience heart failure while receiving sunitinib, effective cardiological management can allow patients to continue oncological treatment and maintain stable cancer disease. However, further studies are required to confirm these findings.
Acknowledgements
Editorial support from ACUMED (Tytherington, UK), with funding from Pfizer Inc. Declaration of interest: Cezary Szczylik is member of advisory boards for Pfizer, Bayer, Schering and Wyeth and that all the other authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007; 356: 115–24
- Figlin, RA, Hutson, TE, Tomczak, P, , et al. Overall survival with sunitinib versus interferon (IFN)-alfa as first-line treatment of metastatic renal cell carcinoma (mRCC). Oral (abstract 5024). Presented at the 44th American Society of Clinical Oncology Annual meeting, Chicago, USA, May, 30–June3, 2008.
- Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomized controlled trial. Lancet 2006; 368: 1329–38
- Schmidinger M, Zielinski CC, Vogl UM, Bojic A, Bojic M, Schukro C, et al. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol 2008; 26: 5204–12
- Force T, Krause DS, Van Etten RA. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat Rev Cancer 2007; 7: 332–44
- Chu TF, Rupnick MA, Kerkela R, Dallabrida SM, Zurakowski D, Nguyen L, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet 2007; 370(9604)2011–19
- Cardinale D, Colombo A, Sandri MT, Lamantia G, Colombo N, Civelli M, et al. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation 2006; 114: 2474–81
- Pfizer Inc . SUTENT, Summary of Product Characteristics. January, 2008. Accessed 23/04/2008. http://emc.medicines.org.uk/emc/industry/default.asp?page=displaydoc.asp&documentid=18531.
- Porta, C, Szczylik, C, Bracarda, S, , et al. Short- and long-term safety with sunitinib in an expanded access trial in metastatic renal cell carcinoma (mRCC). Oral (abstract 5114). Presented at the 44th American Society of Clinical Oncology Annual meeting, Chicago, USA, May, 30–June3, 2008.
- Cooney MM, van Heeckeren W, Bhakta S, Ortiz J, Remiack SC. Drug insight: Vascular disrupting agents and angiogenesis–novel approaches for drug delivery. Nat Clin Pract Oncol 2006; 3: 682–92
- Joensuu H. Cardiotoxicity of sunitinib. Lancet 2007; 370: 1978–9