Abstract
Background. This study systematically evaluates the effects and harms of physiotherapy methods and explores current treatment practices and costs in relation to lymphoedema in breast cancer patients in Finland. Material and methods. A systematic review of randomized controlled trials (RCTs) on physiotherapy interventions for breast cancer patients with lymphoedema. A postal survey to lymph therapists, a telephone and register survey for therapy costs. Results. We identified 14 RCTs, of which two had moderate and the others high risk of bias. There was moderate evidence that compression bandages decreased lymphoedema, and that pneumatic pumps had no effect on lymphoedema. In Finland lymph therapy practice is a combination of manual lymph drainage (MLD), compression bandages, therapeutic exercises and guidance for self-treatment, with an annual average cost of EUR 799 per patient. Conclusions. Compression bandages are likely to reduce upper limb lymphoedema in breast cancer patients. Evidence on other physiotherapy methods and their combinations is limited due to the poor quality of the trials. No evidence was found on any outcomes other than upper limb volume. We call for well-designed trials with patient-related outcomes on the effectiveness of MLD, guidance and therapeutic exercises.
After primary breast cancer treatments the most common chronic impairments are upper limb edema, decreased shoulder mobility, neural tissue injuries causing sensory and motor dysfunctions, and pain of the upper body and limb Citation1–3. The prevalence of lymphoedema varies from 0 to 34% Citation[4], Citation[5]. The prevalence of lymphoedema as well as other upper limb impairments are lower after sentinel lymph node biopsy than after axillary lymph node dissection Citation[4]. Postsurgical irradiation increases the risk of lymphoedema Citation[4]. Other factors that might increase the risk of lymphoedema are obesity, advanced stage of cancer and older age Citation[6]. Also women with postoperative infections and a higher level of postoperative hand use are more likely to have higher incidence and severity of lymphoedema Citation[7]. Participation in regular activity and having good upper body functioning were associated in decreased odds of lymphoedema Citation[5].
The aetiology and pathophysiology of lymphoedema is multi-factorial and not fully understood Citation[8]. In the swollen upper limb there are changes both in the circulation and in the lymphatic systems. Lymphoedema develops when the microvascular filtration rate exceeds lymph drainage; either the filtration rate is high or the lymph flow is low or both these factors are present Citation[9]. As the lymphoedema progresses, collagen deposition may increase, with adipose and connective tissue overgrowth Citation[10]. Visible swelling is detected only after the lymph flow is reduced by 80% Citation[11].
Quality of life has been shown to be lower in breast-cancer-operated women with lymphoedema than for those without it Citation[12]. Lymphoedema can cause activity limitations in home and work environments, and activities like lifting and carrying objects may be difficult Citation[1].
Lymphoedema treatments include compression by garments or bandages (CB) or sleeves (CS) and manual lymphatic drainage (MLD) or a combination of those treatments with advice for skin care and infection prevention. A variety of other methods may also be used e.g. physical therapy modalities, soft tissue mobilisation techniques like massage, and exercise therapy methods like passive or active movements.
In Finland, with a population of 5.3 million, approximately five hundred lymph therapists provide lymphoedema treatments. The Finnish Lymph Therapy Association asked the Finnish Office for Health Technology Assessment (Finohta) to assess the evidence of currently used treatments. There was also a need to clarify the variation and availability of lymph therapy for breast cancer patients in Finland. Full results of the evaluations were reported in Finnish language Citation[13]. This study reports an update of the systematic review of the literature of the Finnish report regarding the effectiveness of physiotherapy methods for treating lymphoedema in breast cancer patients, and describes current lymphoedema therapy practices and costs in Finland.
Material and methods
Systematic review
Literature searches for reviews
We searched for reviews in Ovid Medline, Cinahl, CRD (Centre for Reviews and Dissemination), OAIster (Open Archives Initiative), and PEDro (The Physiotherapy Evidence Database), and the Cochrane Database of Systematic Reviews for the period January 1998 to February 2006. The search terms were “breast cancer” AND “lymphedem” OR “lymphoedem” OR “lymph” OR “edem”. The quality of the reviews was assessed by the Overview Quality Assessment Questionnaire by Oxman Citation[14], Citation[15] complemented with decision rules by Hoving et al. Citation[16]. Given the variations of the identified reviews with regard to research questions, their methodological quality and search periods, it was decided that only original studies would be included.
Literature searches for original studies
To update the searches of the existing reviews, experienced medical librarians utilized the search strategies described in a high-quality review by Pecino et al. 2004 Citation[17]. The search strategy for Medline is available as Appendix A in the web-version of this article. We searched the Ovid Medline, PEDro, Cinahl, and the Cochrane Central Register of Controlled Trials without language restrictions for the period January 2004 to March 2008, and the Embase until November 2007. The reference lists of the identified reviews and included trials were screened for additional references.
Inclusion criteria
Study type: Published full-length articles of randomized controlled trials (RCT).
Population: Breast cancer patients with lymphoedema after cancer treatment.
Interventions: Clinically justifiable physiotherapy methods, as compared to no treatment, placebo, sham therapy, or other physiotherapy (PT) interventions: compression bandages (CB), compression sleeves (CS), manual lymphatic massage or drainage (MLD), mechanical lymphatic massage or drainage, pneumatic compression pump, movement therapy or therapeutic exercise, therapy modalities e.g. laser, heat, electricity, or any combinations of the above. Excluded were surgical and dietary methods and pharmaceutical interventions.
Outcomes: Volume of upper limb, weakness, range of motion restrictions, impairments, increase in functioning or/and work ability, occurrence of soft tissue infections, experienced harms, quality of life. The follow-up periods were categorized as short term (<6 months) and long term (≥6 months).
Language: Danish, English, Finnish, French, German, Norwegian and Swedish.
Study selection, data extraction, and assessment of the methodological quality
Two reviewers (AK and HA) independently screened the search results and selected articles for closer scrutiny. After full texts were ordered, two reviewers (AK and HA or UMR) separately assessed them in relation to the inclusion criteria. Two reviewers (AK and HA or UMR) extracted data on patients, interventions and outcomes, and assessed the quality of the original publications. In case of any disagreement in any phase of this process the third reviewer was involved and agreement was achieved by consensus.
To assess the methodological quality we used internal validity criteria and decision rules modified from Van Tulder et al. Citation[18] (Appendix B, available in web-version) and evaluated the risk of bias according to the Cochrane Handbook Citation[19]. The eleven criteria were related to selection bias, performance bias, attrition bias, and detection bias, and rated as “yes”, “no” or “unclear”. We considered the risk of bias as low when all criteria were fulfilled. Due to the nature of the interventions, blinding of the patient and therapist could not be expected in most studies. If these two criteria and one other criterion were not met the risk of bias was considered as moderate, whereas deficiencies in more than three criteria were considered to constitute high risk of bias.
Survey of current practices and costs
A structured questionnaire was developed together with lymph therapy experts and sent to 258 randomly selected lymph therapists between January and April 2006. The therapist's addresses were obtained from the registers of the Finnish Association of Vodder-lymphtherapists and the Finnish Lymphtherapy Association. The questions concerned origins of the referrals, use and duration of treatments, and pre- and post-therapy assessment methods.
Information on the usage volumes of lymphtherapy and compression bandages was collected from three hospital districts (Helsinki, Pirkanmaa and North Ostro-Bothnia), and three major cities (Helsinki, Tampere and Oulu) in 2005. Prices of compression bandage, sleeves and gloves as well as 60-minute lymph therapy sessions were obtained from service providers and manufactures between January and March 2007.
Data on reimbursed costs for lymph therapy sessions in 2004 were obtained from the national Social Insurance Institution (SII). To identify breast cancer patients from this data, personal identification numbers were used to link the reimbursement data with data from the Finnish Cancer Register. After identification, personal identification numbers were deleted from the study data set. Study permissions were requested and obtained from both organizations.
Synthesis methods
In the systematic review the interventions (type, frequency, duration, and setting) and the outcome measurements differed and therefore the results of the studies could not be combined by meta-analysis. Thus the results were grouped according to the intervention comparisons, intervention types and measured outcomes. The levels of evidence synthesis, presented in , is based on the method of van Tulder et al. Citation[18]. We considered the trials as of high quality when there was a low or moderate risk of bias, and as of low quality when there was a high risk of bias.
Table I. The levels of evidence synthesis Citation[18].
Percentual proportions were calculated from practice survey data by SPSS 14.0. Means and ranges were calculated from cost data.[MSOffice1]
Results
Systematic review
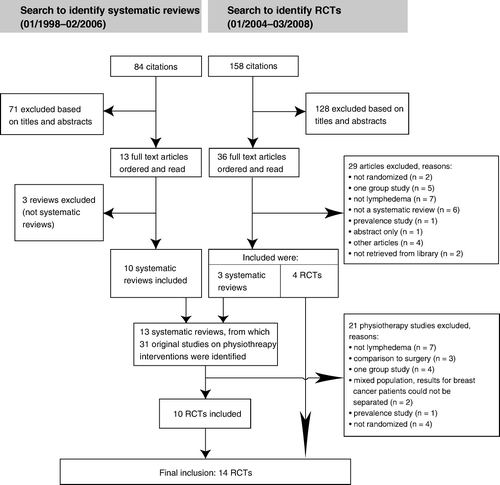
The review search yielded 84 citations from which ten systematic reviews were identified (). From these we identified ten RCTs that fulfilled the inclusion criteria. The update searches for RCTs yielded 158 citations, of which 36 full texts were ordered and read, and four additional RCTs were identified. Thus fourteen RCTs were finally included Citation20–33.
According to our assessment two trials Citation[20], Citation[21] had moderate risk of bias (). Randomization and allocation concealment was adequate in two other trials Citation[22], Citation[23] but the sample size was very small Citation[23], and there was a high risk for performance, detection Citation[22], Citation[23] and attrition biases Citation[22]. Also the other ten trials Citation24–33 had high risk for biased results, as only from 1–6 of the eleven quality criteria were met.
Table II. Methodological quality of the trials (n = 14) Citation[18].
Description of the studies
The studied interventions and comparisons varied across all trials (). Three trials compared an active treatment to no treatment. The active interventions were intermittent compression pumps Citation[21], CS Citation[31] and resistance training Citation[29]. Two trials compared low-level laser to sham treatment Citation[22], Citation[30]. Nine trials compared two different types of treatment, often with CB or CS as an add-on treatment in both groups. In six trials, MLD was compared to shorter therapy sessions and more simplified drainage Citation[23], Citation[32], CB or CS only Citation[20], Citation[25], Citation[26] or to compression pumps Citation[27]. Three trials compared different devices to other devices or CB. Specifically, mechanical compression therapy (Flexitouch) was compared to self-massage Citation[24], electrical stimulation to CS Citation[28], and ultrasound to a compression pump Citation[33] with or without CS.The intervention periods were short ranging from one to four weeks. Most trials had a short-term follow-up: in six trials up to four weeks, and in four trials from one to three months. Long-term follow-up was present in four trials, in two trials for 6 months and in two for one year.
Table III. Effectiveness of different lymph therapy methods on lymphoedema in the 14 original studies.
Decrease in lymphoedema
A decrease in lymphoedema was the main outcome in all trials, although the measurement methods differed across all trials (). The two trials with moderate risk of bias Citation[20], Citation[21] reported no between-group differences as detailed below.
Only one trial with moderate risk of bias Citation[20] compared MLD with CB to CB only (). The patients (aged 45 – 76 years) had one breast and the axillary nodes removed. At baseline the treatment group (n = 25) arm volume was 695±696 ml and the control group (n = 25) 672±672 ml. After four weeks, lymphoedema decreased equally in both groups, 260±217 ml (p < 0.001) in MLD with the CB group and 246±159 ml (p < 0.001) with CB only. In most patients lymphoedema had reduced already within the first two weeks. The between-group difference was only 14 ml, 3% showing no statistical difference (95% CI 103–301 ml, p = 0.812). According to the results of this moderate quality trial there is moderate evidence that MLD has no additional effect on the reduction of upper limb volume achieved with CB alone.
In another trial with moderate risk of bias Citation[21], pneumatic compression was compared to no treatment (). In this trial the breast cancer patients (aged 52 – 74 years) had partial or whole removal of one breast and axillary nodes. The treatment by intermittent pneumatic compression pump (60 Hgmm) was scheduled for two 2-week periods, with a 5-week break between the periods. Advice on skin care and infection prevention was given to both groups. At baseline, patients in the pneumatic compression group (n = 40) had more lymphoedema (16.1±5.4 cm) than in the no-treatment group (14.6±4.4 cm) (n = 40). Thirteen patients (16%) dropped out. Measured with a summary of circumference measurements at nine sites, the mean decrease of lymphoedema was 1.9 cm in the index group and 0.5 cm in the no-treatment group. A decrease of over 25% of lymphoedema was obtained in equal proportions of patients for both groups. There was no between-group difference (p = 0.08). Thus this trial with moderate risk of bias shows moderate evidence that pneumatic compression has no effect on lymphoedema.
The other twelve trials had high risk of bias, which means that the results of these trials are far less trustworthy. These low-quality trials had somewhat contradictory results. Eight trials showed no between-group differences in the decrease of lymphoedema (). In four trials Citation[25], Citation[31], Citation[32] some differences between the groups were observed. However, these trials had inherent biases in reporting the results: the results were not analyzed statistically Citation[31], the method for calculating the results was not reported Citation[25], the group difference before the cross-over was not given Citation[32] or the results were not analyzed in the original groups Citation[24].
Ten trials had CB or CS as an add-on treatment in both groups. These trials observed a 4 – 60% decrease of lymphoedema in all those groups having CB or CS as an add-on treatment, even though they did not find any between-group differences, no matter the comparison. These multiple low-quality trials may provide some evidence from within-group comparisons i.e. from before and after treatment measurements, to support the finding above that compression garments may be enough to reduce upper limb lymphoedema.
Only one trial on electrical stimulation Citation[28] reported on adverse events. The amount of lymphoedema increased more than 25% during the intervention in five patients (14%) in the treatment group and one patient (3%) in the control group and these patients were excluded from the final analyses. Thus there is limited evidence that electrical stimulation may be harmful, by causing an increase in the amount of edema, for breast cancer patients with lymphoedema.
Other outcomes
No trials measured patient-related outcomes as a primary outcome, e.g. adverse events, quality of life or functional ability. In some trials pain, upper limb functioning, work ability, quality of life, daily activities, weight, axillary edema and range of motions of upper limb were used as secondary outcomes Citation[22], Citation24–27, Citation29–33. There was no systematic way of using these measures and the results were mainly not reported numerically or the between-group differences were not analyzed and tested statistically. Thus there is no evidence on whether any of the studied interventions have any benefits other than the reduction of the upper limb volume.
Survey
Questionnaires were returned by 178 lymph therapists (69% response rate). Of these, 106 reported that they treat breast cancer patients. Lymph drainage therapy consisted of a combination of MLD (99%), guidance (79%), CS (74%), CB (63%), and therapeutic exercises (55%). Pneumatic pumps were used only by two therapists and electrical devices by none. Most therapists (80%) used 60-minute sessions, most commonly (66%) in a series of 11 to 15 sessions.
Referrals for lymph therapy were mostly received from physicians working at health centers or in occupational healthcare (38%), or at cancer (30%) or surgery (17%) departments in hospitals. The sources of funding were variable: municipalities (33%), patients (being reimbursed by the SII) (32.4%), hospital district (19%), the SII (6.7%), and others (12.3% e.g. insurance company, employer).
The SII reimbursed the costs of lymph therapy to 547 patients, with a mean therapy cost of EUR 467 (compression garments not necessarily included) in 2004. The estimated costs for lymph therapy treatment based on 2007 prices are shown in . If a patient receives annually ten therapy sessions with compression bandages, and two compression sleeves and gloves, the mean annual therapy cost would be EUR 799. By combining the survey information and the SII data, we calculated that some 1 800 patients are receiving treatment for lymphoedema annually in Finland. Thus the mean annual cost of combination therapy sums to EUR 1.4 million (range 0.8 to 2.0 million). This calculation does not include the costs of therapy offered by therapists working at the publicly funded city hospitals.[MSOffice2]
Table IV. The costs of lymph therapy (mean, ranges) in euros per patient in 2007.
Discussion
This study systematically analysed the effects and harms of lymph therapy treatments and compared them to current treatment practices and costs in Finland. We identified and analyzed 14 RCTs and collected information from lymph therapists on treatment methods used on breast cancer patients. Therapy costs were obtained from available sources. The results may be utilized in evidence-based decision-making and planning for further research in this field.
Previously, eleven reviews have evaluated the effectiveness of various lymphoedema treatments. Unlike these reviews, we included only randomized trials, as these are widely considered to offer the best designs for protecting against various systematic biases. Furthermore, we focused only on physical therapy methods on breast cancer patients with lymphoedema. The search update identified four trials Citation[20], Citation[24], Citation[25], Citation[30] that have not been analyzed in previous reviews. Surprisingly, no RCTs were identified from German-speaking parts of Europe, where many of these therapies have been developed and are widely used.
Overall, the methodological quality of the trials was poor. Only two trials Citation[20], Citation[21] had a moderate risk of bias. Thus, we could establish only moderate evidence on some interventions on reduction of lymphedema. Based on one trial CB reduces lymphoedema, but adding MLD does not bring any additional effect. This finding may be supported by the observations in other ten studies in which the within-group decrease of lympoedema was present in all those groups that used compression as an add-on treatment. Based on one trial there was also moderate evidence that pneumatic compression has no effect on lymphoedema. The evidence of any other single treatment method or their combination is limited because only low-quality trials were available. No studies were found on the effects of the combination therapies (MLD, CB, therapeutic exercises, guidance) that are most in use according to our survey of current practices in Finland. This evidence base will hopefully improve in the near future, as there are at least six ongoing RCTs on MLD, compression and therapeutic exercises in the trial registers of the UK (www.controlled-trials.com) and the USA (www.clinicaltrials.gov).
Only one trial reported on harms or adverse effects of the studied treatments but left out of the final analysis those patients whose lymphoedema had increased because of the treatment Citation[28]. As new devices are constantly being developed, it is important to evaluate their effectiveness and harms rigorously before implementation to patient care.
All trials used the reduction of lymphoedema as the main outcome, but measured it in different ways. To allow comparisons across trials measurement methods need to be harmonised. Furthermore, patient-related outcomes should also be measured as therapy aims are often multimodal. We suggest measuring range of motion restrictions, occurrence of soft tissue infections, overall functioning, work-ability, experienced harms, and quality of life, that have already been utilized in observational studies Citation34–38.
The Finnish survey cost data may be used to compare and discuss therapy practices at an international level. In Finland the therapists typically combine the modalities: MLD, CB, therapeutic exercises and guidance for self-treatment. The MLD was the most dominant part of the therapy session, whereas compression garments are not necessarily used. The reasons for not applying compression garments were not elicited in our survey questionnaire, but low compliance with wearing a CB, and higher motivation in making use of lymph massage have been reported in other studies Citation[39]. As the evidence emphasizes the importance of compression, the lymph therapists really should motivate patients to make use of this treatment.
Overall, the survey results clearly show the clinical reality, which does not fit with the evidence base of the studies carried out internationally. The results of a previous version of this review were reported in a Finnish language report in 2007 Citation[13] that was distributed widely to all Finnish lymph therapists and cancer and surgery departments treating breast cancer patients. It was discussed in two national seminars. The overall impression in the seminars was that many lymph therapists have been unaware of the evidence-base of the treatments used, particularly of the poor additional effect of MLD in addition to compression garments. Consequently, both lymph therapy associations have changed their training to emphasize the use of compression as the crucial part of treatment. The lymph therapists should seriously think about increasing their awareness and also take their own steps to build the evidence base through rigorous and well-designed research settings.
Even though our searches were rather comprehensive, there is a possibility that we have not identified all trials because of e.g. publication bias. Another explanation may be that trials in this field still await publication or implementation. Another limitation of this review may be that we did not include observational designs which have evaluated longer follow-up periods. For example, a recent study followed 537 patients prospectively for one year and showed that intensive decongestive therapy results can be maintained by daily use of elastic sleeve (daytime) and low stretch bandage (at night) Citation[40]. Non-compliance with CB and CS were risk factors for increased lymphoedema, but non-compliance with MLD was not, which is consistent with the findings by McNeely et al. Citation[20]. Long term observational studies with consistent association and no plausible confounders can add to the evidence base Citation[41], and should perhaps be included in future systematic reviews.
The knowledge of the methodological flaws in the current trials can be utilized in designing future studies in this field. The researchers should carefully plan and implement randomization, conceal the group allocation and analyze results in the groups that were formed by the randomization. The known prognostic factors (age, operation type, postoperative treatment, amount of lymphoedema and severity of the oedema, previous treatment and use of the compression garments) should be reported as baseline characteristics. Furthermore, sample size calculation is needed. All trials in this review were quite small, thus there is a real risk that none of these studies were powered enough to detect real differences between the groups.
From a patient's functional perspective, the aim of lymph therapy is to reduce lymphoedema and maintain or increase the patient's activity and participation. Lymphoedema treatment, however, still lacks much of evidence-base and yet it is associated with significant investments of time and financial resources. The available evidence suggests that compression bandages are likely to reduce upper limb lymphoedema in breast cancer patients. Limited or no evidence was found of the effect of other physiotherapy methods and their combinations on lymphoedema or any other outcomes. We call for well-designed trials to study the effectiveness of MLD, guidance and therapeutic exercises using patient-related outcomes.
Acknowledgements
This study was funded by Finohta, a national government-funded organization for health technology assessment in Finland. The authors thank information scientists Ulla Neuvonen from the National Library for Health Sciences, Leena Raustia MSc and Jaana Isojärvi MSc from Finohta for their support in the literature search, and Mark Phillips BA for his help in polishing the language of the article. Special thanks for the co-operation in collecting the survey data goes to the Finnish Association of Vodder-lymphtherapists, the Finnish Lymphtherapy Association, the Health Centers and the University Hospitals in Helsinki, Tampere and Oulu, the private lymph therapy service providers, the therapy equipment importers and to Linda Akiola for organizing data collection and entering the survey data.All authors designed the study protocol. AK, HA and UMR extracted the data and assessed the study quality of the trials. All authors analyzed the results. AK and HA wrote the manuscript. TT and UMR provided critical revisions to it. All authors read and approved the final manuscript. Declaration of interest: The authors declare that they have no conflicts of interests.
References
- Kärki A, Simonen R, Mälkiä E, Selfe J. Impairments, activity limitations and participation restrictions 6 and 12 months after breast cancer operation. J Rehab Med 2005; 37: 180–8
- Gossenlink R, Rouffaer L, Vanhelden P, Piot W, Trooster T, Christiaens M. Recovery of upper limb function after axillary dissection. J Surg Oncol 2003; 83: 204–11
- Rietman JS, Dijkstra PU, Geertzen JH, Baas P, de Vries J, Dolsma WV, et al. Treatment-related upper limb morbidity 1 year after sentinel lymph node biopsy or axillary lymph node dissection for stage I or II breast cancer. Ann Surg Oncol 2004; 11: 1018–24
- Lee T, Kilbreath S, Refshauge K, Herbert R, Beith J. Prognosis of the upper limb following surgery and radiation for breast cancer. Breast Cancer Res Treat 2008; 110: 19–37
- Hayes SC, Janda M, Cornish B, Battistutta D, Newman B. Lymphedema after breast cancer; incidence, risk factors, and effect on upper body function. J Clin Oncol 2008; 26: 3536–42
- Rönkä, R. Feasibility of sentinel node biopsy in breast cancer surgery [Dissertation]. University of Helsinki: Helsinki; 2004.
- Soran A, D'Angelo G, Begovic M, Ardic F, Harlak A, Samuel Wieand H, et al. Breast cancer-related lymphedema – what are the significant predictors and how they affect the severity of lymphedema?. Breast J 2006; 12: 536–43
- Lane K, Worsley D, McKenzie D. Exercise and the lymphatic system, implications for breast-cancer survivors. Sports Med 2005; 35: 461–71
- Mortimer P, Levick R. Chronic peripheral oedema: The critical role of the lymphatic system. Clin Med 2004; 4: 448–53
- Rockson S. Lymphedema. Am J Med 2001; 110: 288–95
- Clodius L. Lymphedema common sense. Eur J Plast Surg 2002; 25: 66–80
- Velanovich V, Szymanski W. Quality of life of breast cancer patients in lymphedema. Am J Surg 1999; 177: 184–7
- Anttila, H, Kärki, A, Rautakorpi, U-M. Lymfaturvotuksen fysioterapia rintasyöpäpotilailla. Vaikuttavuus, käytännöt ja kustannukset [Lymphoedema therapy in breast cancer patients. Effectiveness, current practices and costs]: Finohta's report. 30; 2007.
- Oxman, AD, Guatt, GH, Dinger, J, et al. Agreement among reviewers of review articles. J Clin Epidemiol 1991:91–8.
- Oxman, AD, Guatt, GH. Validation of an index of the quality of review articles. J Clin Epidemiol 1991:1271–8.
- Hoving JL, Gross AR, Gasner D, Kay T, Kennedy CH, Hondres MA, et al. A critical appraisal of review articles on the effectiveness of conservative treatment for neck pain. Spine 2001; 26: 196–205
- Pecino, F, Pérez de la Blanca, E, Gago, T. Eficacia de la fisioterapia para el tratamiento del linfedema asociado a mastectomía: Agencia de Evaluación de Tecnologías Sanitarias de Andalucía. 2004. Report No.: 12.
- van Tulder, M, Furlan, A, Bombardier, C, Bouter, LM,. the Editorial Board of the Cochrane Collaboration Back Review Group. Updated method guidelines for systematic reviews in the Cochrane Collaboration Back Review Group. Spine, 2003;28:1290–9.
- Higgins, JPT, Green, S, Cochrane handbook for systematic reviews of interventions. Version 5.0.0 [updated February 2008]. Accessed: May 19th 2008. Internet: www.cochrane-handbook.org. 2008.
- McNeely ML, Magee DJ, Lees AW, Bagnall KM, Haykowsky M, Hanson J. The addition of manual lymph drainage to compression therapy for breast cancer related lymphedema: A randomized controlled trial. Breast Cancer Res Treat 2004; 86: 95–106
- Dini D, Del Mastro L, Gozza A, Lionetto R, Garrone O, Forno G, et al. The role of pneumatic compression in the treatment of postmastectomy lymphedema. A randomized phase III study. Ann Oncol 1998; 9: 187–90
- Carati CJ, Anderson SN, Gannon BJ, Piller NB. Treatment of postmastectomy lymphedema with low-level laser therapy: A double blind, placebo-controlled trial. Cancer 2003; 98: 1114–22
- Sitzia J, Sobrido L, Harlow W. Manual lymphatic drainage compared with simple lymphatic drainage in the treatment of postmastectomy lymphoedema. Physiotherapy 2002; 88: 99–107
- Wilburn O, Wilburn P, Rockson SG. A pilot, prospective evaluation of a novel alternative for maintenance therapy of breast cancer-associated lymphedema. BMC Cancer 2006; 6: 84
- Didem K, Ufuk YS, Serdar S, Zumre A. The comparison of two different physiotherapy methods in treatment of lymphedema after breast surgery. Breast Cancer Res Treat 2005; 93: 49–54
- Andersen L, Hojris I, Erlandsen M, Andersen J. Treatment of breast-cancer-related lymphedema with or without manual lymphatic drainage–a randomized study. Acta Oncol 2000; 39: 399–405
- Johansson K, Lie E, Ekdahl C, Lindfeldt J. A randomized study comparing manual lymph drainage with sequential pneumatic compression for treatment of postoperative arm lymphedema. Lymphology 1998; 31: 56–64
- Bertelli G, Venturini M, Forno G, Macchiavello F, Dini D. Conservative treatment of postmastectomy lymphedema: A controlled, randomized trial. Ann Oncol 1991; 2: 575–8
- McKenzie DC, Kalda AL. Effect of upper extremity exercise on secondary lymphedema in breast cancer patients: A pilot study. J Clin Oncol 2003; 21: 463–6
- Kaviani A, Fateh M, Nooraie RY, Alinagi-Zadeh M, Ataie-Fashtami L. Low-level laser therapy in management of postmastectomy lymphedema. Lasers Med Sci 2006; 21: 90–4
- Hornsby R. The use of compression to treat lymphoedema. Prof Nurse 1995; 11: 127–8
- Williams AF, Vadgama A, Franks PJ, Mortimer PS. A randomized controlled crossover study of manual lymphatic drainage therapy in women with breast cancer-related lymphoedema. Eur J Cancer Care (Engl) 2002; 11: 254–61
- Balzarini A, Pirovano C, Diazzi G, Olivieri R, Ferla F, Galperti G, et al. Ultrasound therapy of chronic arm lymphedema after surgical treatment of breast cancer. Lymphology 1993; 26: 128–34
- Tobin M, Lacey H, Meyer L, Mortimer P. The psychological morbidity of breast cancer -related arm swelling. Lymphoedema 1993; 72: 3248–52
- Kärki A, Simonen R, Mälkiä E, Selfe J. Postoperative education concerning the use of the upper limb, and exercise and treatment of the upper limb: A cross-sectional survey of 105 breast cancer patients. Support Care Cancer 2004; 12: 347–54
- Segeström K, Bjerle P, Nyström Å. Importance of time in assessing arm and hand function after treatment of breast cancer. Scand J Plast Reconstr Hand Surg 1991; 25: 241–4
- Oxman, AD, Guatt, GH. Validation of an index of the quality of review articles. J Clin Epidemiol 1991:1271–8.
- Armer J, Radina M, Porock D, Culbertson S. Predicting breast cancer-related lymphedema using self-reported symptoms. Nurs Res 2003; 52: 370–9
- Bani HA, Fasching PA, Lux MM, Rauh C, Willner M, Eder I, et al. Lymphedema in breast cancer survivors: Assessment and information provision in a specialized breast unit. Patient Educ Couns 2007; 66: 311–8
- Vignes S, Porcher R, Arrault M, Dupuy A. Long-term management of breast cancer-related lymphedema after intensive decongestive physiotherapy. Breast Cancer Res Treat 2007; 101: 285–90
- Oxman, A, for the GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490–4.
Appendix A:
Search strategy for Medline, modified from Pecino et al Citation[17].
exp Mastectomy/
exp Mastectomy/ae, mo, cl, nu, ct, px, ec, rh, ed, st, hi, sn, is, td, lj, ut, mt, ve [Adverse Effects, Mortality, Classification, Nursing, Contraindications, Psychology, Economics, Rehabilitation, Education, Standards, History, Statistics & Numerical Data, Instrumentation, Trends, Legislation & Jurisprudence, Utilization, Methods, Veterinary]
1 not 2
exp Mastectomy/ae, rh [Adverse Effects, Rehabilitation]
3 or 4
Breast Neoplasms/
Breast Neoplasms/bl, mi, bs, mo, cf, nu, ci, ps, ch, pa, cl, pp, co, pc, cn, px, di, ra, dh, ri, dt, rt, ec, rh, em, sc, en, se, ep, su, eh, th, et, us, ge, ul, hi, ur, im, ve, me, vi [Blood, Microbiology, Blood Supply, Mortality, Cerebrospinal Fluid, Nursing, Chemically Induced, Parasitology, Chemistry, Pathology, Classification, Physiopathology, Complications, Prevention & Control, Congenital, Psychology, Diagnosis, Radiography, Diet Therapy, Radionuclide Imaging, Drug Therapy, Radiotherapy, Economics, Rehabilitation, Embryology, Secondary, Enzymology, Secretion, Epidemiology, Surgery, Ethnology, Therapy, Etiology, Ultrasonography, Genetics, Ultrastructure, History, Urine, Immunology, Veterinary, Metabolism, Virology]
6 not 7
Breast Neoplasms/co, rh, su [Complications, Rehabilitation, Surgery]
8 or 9
Lymph Node Excision/
Lymph Node Excision/ae, mo, cl, nu, ct, px, ec, rh, ed, st, es, sn, hi, td, is, ut, mt, ve [Adverse Effects, Mortality, Classification, Nursing, Contraindications, Psychology, Economics, Rehabilitation, Education, Standards, Ethics, Statistics & Numerical Data, History, Trends, Instrumentation, Utilization, Methods, Veterinary]
11 not 12
Lymph Node Excision/ae, rh [Adverse Effects, Rehabilitation]
13 or 14
5 or 10 or 15
Lymphedema/nu, pc, px, rh, th [Nursing, Prevention & Control, Psychology, Rehabilitation, Therapy]
Lymphedema/
physical-therapy-techniques/
massage/
exercise-therapy/
bandages/
exp drainage/
surgical procedures, operative/ or exp suction/
23 not 24
pressure/
Posture/
Posture/ph [Physiology]
27 not 28
movement/
Movement/de, re, ph [Drug Effects, Radiation Effects, Physiology]
30 not 31
19 or 20 or 21 or 22 or 25 or 26 or 29 or 32
18 and 33
LYMPHEDEMA.hw.
16 and (17 or (35 and 33))
(foldi or vodder or casley-smith or lerner or leduc).ti.
37 and (16 or 17)
(lymphedema or lymphoedema or lymphoedematous or oedema).ti.
(mastectomy or post?mastectomy or lumpectomy or breast saving operation or breast conserving treatment or breast cancer).ti,ab.
(physiotherapy or drainage or bandag$ or exercise or “physical therapy” or decongestive or pressure or compression or rehabilitation or management).ti.
39 and 40 and 41
36 or 38 or 42
(letter or editorial).pt.
43 not 44
randomized-controlled-trial.pt.
controlled-clinical-trial.pt.
randomized-controlled-trials/
random-allocation.mp.
double-blind-method.mp.
single-blind-method.mp.
46 or 47 or 48 or 49 or 50 or 51
clinical-trial.pt.
exp clinical-trials/
(clin$ adj trial$).ti,ab.
((singl$ or doubl$ or trebl$ or tripl$) adj (blind$ or mask$)).ti,ab.
clin.ti,ab.
placebos/
placebo$.ti,ab.
random$.ti,ab.
research-design/
53 or 54 or 55 or 56 or 57 or 58 or 59 or 60
limit 62 to comparative study
exp evaluation-studies/
follow-up-studies.mp.
prospective studies.mp.
(control$ or prospectiv$ or volunteer$).ti,ab.
63 or 64 or 65 or 66 or 67
52 or 62 or 68
limit 69 to humans
45 and 70
limit 71 to yr = “2004-2008”
Appendix B:
Quality assessment criteria and decision rules adapted and modified from van Tulder et al Citation[18].
Criteria list
Was the method of randomization adequate?
Was the treatment allocation concealed?
Were the groups similar at baseline regarding the most important prognostic indicators?
Was the patient blinded to the intervention?
Was the care provider blinded to the intervention?
Was the outcome assessor blinded to the intervention?
Were co-interventions avoided or similar?
Was the compliance acceptable in all groups?
Was the drop-out rate described and acceptable?
Was the timing of the outcome assessment in all groups similar?
Did the analysis include an intention-to-treat analysis?
Decision rules
A random (unpredictable) assignment sequence. Examples of adequate methods are computer-generated random number table or similar. Methods of allocation using date of birth, date of admission, hospital numbers, or alternation should not be regarded as appropriate.
Assignment generated by an independent person not responsible for determining the eligibility of the patients. This person has no information about the persons included in the trial and has no influence on the assignment sequence or on the decision about eligibility of the patient.
In order to receive a “yes,” groups have to be similar regarding prognostic factors at baseline (age, surgery method, radiation therapy) and value of main outcome measure (amount of lymphoedema).
The reviewer determines if enough information about the blinding is given in order to score a “yes.”
Co-interventions should either be avoided in the trial design or similar between the index and control groups.
The reviewer determines if the compliance to the interventions is acceptable, based on the reported intensity, duration, number and frequency of sessions for both the index intervention and control intervention(s).
No dropouts; or the number of participants who were included in the study but did not complete the observation period or were not included in the analysis must be described and reasons given. If the percentage of withdrawals and drop-outs does not exceed 20% for short-term follow-up and 30% for long-term follow-up and does not lead to substantial bias a “yes” is scored.
Timing of outcome assessment should be identical for all intervention groups and for all important outcome assessments.
All randomized patients are reported/analyzed in the group they were allocated to by randomization for the most important moments of effect measurement (minus missing values) irrespective of noncompliance and co-interventions.