Abstract
Background. The aims of the current explorative study were to assess persistent neuropathy in 45 patients up to 6 years after treatment with cisplatin or oxaliplatin and to determine the most adequate method to evaluate neuropathy. Furthermore, the effect of possible determinants on persistent neuropathy was investigated. Material andmethods. The assessment of neuropathy was performed using a questionnaire, by neurological tests, and by vibration threshold (VT) measurements. Because VT determination gives the most objective information, VT measurements were used for further analyses. Results and discussion. The analyses revealed that neuropathy of the hands was related to follow-up time, with an observed recovery half-life of 6.8 (± 3.1) years. No significant reversibility of neuropathy of the feet within the observation period could be demonstrated. For cisplatin, the severity of neuropathy was related to the cumulative dose and sodium thiosulfate use. Oxaliplatin induced neuropathy did not appear to be related to the dose within the studied dose range. No relationship with platinum levels, renal function, glutathione transferase genotypes, diabetes mellitus, alcohol use, or co-medication could be demonstrated. This study was performed as an explorative study and the issues discussed need to be investigated further.
Cisplatin belongs to one of the most frequently used chemotherapeutics and is applied extensively in the treatment of several tumour types. Chemotherapy with cisplatin containing regimens is, however, often accompanied by severe side effects, such as nephrotoxicity, ototoxicity, and peripheral sensory neuropathy. The search for platinum (Pt) anticancer agents with less severe side effects and increased efficacy has led to the development of several Pt-based compounds, including oxaliplatin. Oxaliplatin was first introduced into clinical trials in 1986 Citation[1] and is now part of the standard first-line treatment in patients with colorectal cancer Citation2–4. Unfortunately, patients treated with this compound also frequently suffer from persistent peripheral sensory neuropathy. Considering the fact that the life expectancy of patients treated with cisplatin and oxaliplatin has improved, persistent side effects, such as neuropathy, can greatly affect the quality of life of patients.
Clinical manifestations of cisplatin and oxaliplatin induced persistent neuropathy are primarily sensory in nature Citation[5]. Parasthesia and dysesthesia in the hands and feet are the most prominent symptoms Citation[4], Citation[6]. The occurrence of L'hermitte's sign is also reported, indicating the involvement of the dorsal columns of the spinal cord Citation7–9. For cisplatin, it is mentioned that symptoms often occur or increase after completion of treatment Citation[10] with off-therapy deterioration up to approximately 6 months after withdrawal of cisplatin Citation[7], Citation[8]. In general, symptoms tend to recover slowly and the process is often incomplete Citation[6]. Oxaliplatin induced persistent neuropathy has been less well characterised. Long-term follow-up is difficult because the prognosis of patients treated with this drug is often less than 20 months Citation[11]. It has, however, been mentioned that the recovery from oxaliplatin neuropathy is faster and more complete than recovery from cisplatin neuropathy Citation[6].
Many studies have been performed to unravel the mechanism behind Pt-induced persistent neuropathy, but still, the exact mechanism has not been clarified. Platinum agents seem to target a variety of structures and functions in the peripheral nervous system Citation[12]. The dorsal root ganglion appears to be the primary site at which neural damage occurs. For cisplatin, it was shown that Pt was retained in the dorsal root ganglia of patients who were analysed post mortem Citation[13], Citation[14] and that there were morphological changes visible in these ganglia following treatment Citation[13], Citation[15].
Despite the hypotheses on the mechanisms of Pt-induced sensory neuropathy, it still is not fully elucidated why neuropathy has such a chronic character. It was suggested that neuropathy may be persistent due to an irreversible damage at the time of chemotherapy or due to a persistent Pt binding in the dorsal root ganglia.
The evaluation of sensory neuropathy is complicated by the subjective nature of most assessment methods. Several approaches for the determination of the presence and severity of neuropathy have been used in the past, among which were clinical neurological examinations Citation[7], Citation[11], Citation[16], nerve conduction studies Citation[7], questionnaires Citation[17], Citation[18], and the determination of the vibration threshold (VT measurements) Citation[11], Citation[19], Citation[20]. Assessment of neuropathy by these methods has usually been done during or shortly after cessation of treatment.
The aims of the current explorative study were to assess persistent neuropathy in 45 patients more than eight months after treatment with cisplatin or oxaliplatin and to determine the most adequate method to evaluate neuropathy. The assessment of neuropathy was done using a questionnaire and neurological tests. Besides, neuropathy was evaluated quantitatively by vibration threshold (VT) measurements. In addition, we investigated possible determinants of persistent neuropathy, such as Pt agent, follow-up time (time from end of therapy until inclusion in the current study), cumulative dose, plasma Pt levels, age, route of administration, renal function, glutathione S-transferase (GST) genotypes, co-administration of calcium/magnesium with oxaliplatin or sodium thiosulfate (STS) with intra-arterial cisplatin, co-medication, and co-morbidity (alcoholism and diabetes).
Material and methods
Patients
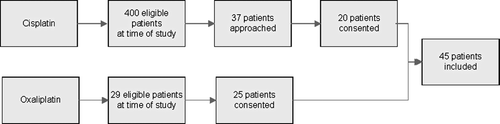
For cisplatin, patients were selected at random from all patients who started treatment between 2000 and 2004, received cumulative cisplatin doses of ≥ 300 mg/m2, and were available for follow-up. For this pilot study, 20 patients of the 400 eligible patients were included. To select the patients, random selections were performed on the 400 eligible patients. From the randomly generated inclusion request to 37 patients, 20 patients consented.
SPSS (SPSSinc, version 11.0, Chicago, IL, USA) was used for random sample selection. For oxaliplatin, all available patients who started treatment between 2000 and 2005 and received cumulative oxaliplatin doses of ≥ 585 mg/m2 were approached for participation in the current study. Of the requested 29 patients, 25 patients consented. A flow chart of the patient inclusion is depicted in . Patients who received treatment with other chemotherapeutic agents that may induce neurotoxicity itself such as vinca alkaloids and taxanes were excluded from the study. The Medical Ethics Committee of the hospital approved the study protocol and all patients gave their written informed consent.
Evaluation of nerve function
Neuropathic symptoms were assessed qualitatively using a questionnaire. The questionnaire was composed of items from previously applied questionnaires which were specifically associated with chemotherapy induced persistent sensory neuropathy Citation[21] and persistent Pt-induced neuropathy Citation[17]. Questions addressed sensory symptoms in the upper (10 questions) extremities (hands), lower (7 questions) extremities (feet), and the orofacial area (3 questions). Questionnaire subjects are summarised in . The patients were asked to answer all questions. The severity of the symptoms was graded as 0 (patient did not suffer from symptoms at all), 1 (patient suffered from symptoms a little), 2 (patient suffered from symptoms pretty much), and 3 (patient suffered from symptoms very much). When all the questions were answered with a score 0, patients were considered as not having clinical neuropathy. Patients who answered one or more questions with a score of 1 or more were designated as suffering from clinical neuropathy. In addition to this dichotomous scaling, the scores of the individual questions for each location (hands, feet, and orofacial area) were summed to get an impression of the severity of the neuropathy. A sum-score of 0 indicated the absence of clinical neuropathy.
Table I. Questionnaire items
The sensory nerve function was also assessed by two neurological balance tests: the Sensitized Romberg Test (SRT) and Tandem Gait (TG). The SRT was used to determine how long a patient was able to stand steady approximated (the toes of one foot touching the heel of the foot in front of it), eyes open and then closed. The length was recorded in seconds. More than ten seconds was considered as normal balance (0), whereas less than ten seconds was considered as abnormal balance (1). The TG was used to determine if a patient was capable to walk on a straight line with the heel of the first foot touching the toes of the foot behind it. The results of the TG were recorded as normal stability (0), difficulty to remain stable, but still able to perform TG (1) and tendency to fall, not able to perform TG (2).
Neuropathy was assessed quantitatively by vibration threshold (VT) determination Citation[22], Citation[23] using a Vibrameter type IV device (Somedic AB, Stockholm, Sweden). Measurements were performed in triplicate at the dorsum of the metacarpal bone of the right and left index finger and of the dorsomedial aspect of the first metatarsal bone of the right foot. The VT was recorded as micrometers of skin displacement and was assessed using the method of limits, which showed an acceptable measure of reproducibility and validity Citation[22], Citation[24], Citation[25]. All VT measurements were determined by the same investigator to prevent inter-observer variability.
The dichotomous results from the questionnaires and VT method were compared and the questionnaire was used to select an optimal cut-off value for the VT method. Furthermore, the questionnaire sum-scores were compared to the continuous VT measurements to see whether the VT measurements could predict the patients’ experience of neuropathy. Besides, the associations between questionnaire sum-scores, VT measurements, and neurological tests were evaluated.
Genotyping
Blood samples were drawn in 5 mL edta-containing tubes (Becton Dickinson Vacutainer Systems, Plymouth, UK) for genotyping of detoxifying GST enzymes. Lymphocyte DNA was isolated according to the method of Boom Citation[26]. All samples were stored at −20°C until analysis. Polymorphisms in the genes encoding the enzymes GSTM1, GSTT1, and GSTP1 were determined. In GSTT1 and GSTM1, known inherited homozygous deletions are equivalent to nonfunctional enzymes and are encoded as positive and negative Citation[27]. In the GSTP1 gene, a functional SNP between adenosine (A) and guanosine (G) at base pair 313 leads to the expression of either Ile or Val at codon 105. This polymorphism significantly affects enzyme activity Citation[28].
PCR amplifications were performed in 50 µL reactions with ~100 ng of genomic DNA, 200 µM dNTPs (Epicentre Technologies, Madison, WI, USA), 10 x PCR Buffer II (Applied Biosystems, Foster City, CA, USA), magnesiumchloride (MgCl2), 0.5 – 1 U AmpliTaq Gold (Applied Biosystems), and forward and reverse primers (Metabion, Planegg-Martinsried, Germany). GSTM1 and GSTT1 deletions were analysed using a gel electrophoresis method with β-globulin as internal control as described by Sreelekha et al. Citation[29]. GSTP1 (exon 5) was genotyped according to Jerónimo et al. Citation[30].
Clinical parameters
Data regarding determinants that might affect sensory nerve function were collected. Cumulative dose of the Pt agents, age, follow-up time, route of administration, co-administration of calcium/magnesium or STS, co-morbidity e.g. alcoholism and diabetes, co-medication, and serum creatinine before start of chemotherapy were collected from patient files. Additionally, serum creatinine was assessed at the time of study. The glomerular filtration rate (GFR) was estimated from serum creatinine using the ‘Modification of Diet in Renal Disease (MDRD)’-formula Citation[31]. Plasma Pt levels at the time of follow-up were described in a previous investigation Citation[32].
Statistical analyses
The Cronbach's alpha coefficient was used to test the internal consistency of the questionnaire using SPSS. It is allowed to use a sum-score when alpha values higher than 0.70 were achieved Citation[33]. Receiver operating characteristic curves (ROC curves) were used to assess the sensitivity and specificity of the VT method. ROC curves were composed using SPSS. Boxplots (SPSS) were used to evaluate the association between VT values and neurological tests. Possible relationships between determinants and neuropathy were evaluated using non-linear mixed effects modeling using NONMEM software (Version V1) (GloboMax LLC, Ellicott city, MD, USA). The first order conditional estimation method was used throughout. It was assumed that, due to the long follow-up, the treatment period was negligible compared to the follow-up time. The significance of established relationships was assessed using the likelihood ratio test.
Results
Patients
summarises the characteristics of the participants. Participants were treated with cisplatin for diverse tumour types, whereas all patients treated with oxaliplatin were diagnosed with colorectal cancer. Five participants were treated with STS in combination with 600 mg/m2 intra-arterially administered cisplatin. For oxaliplatin, 24 of 25 participants received co-administration of Ca/Mg. The range in the follow-up time of patients was between 8 and 75 months.
Table II. Characteristics of participants
Scores of questionnaire and neurological tests
The questionnaire revealed that 26 patients (11/20 for cisplatin (55%), 15/25 for oxaliplatin (60%)) were classified as experiencing symptoms of sensory neuropathy in the hands. Neuropathy in the feet was experienced by 32 patients (9/20 for cisplatin (45%), 23/25 oxaliplatin (92%)). No patients showed orofacial symptoms. The Cronbach's alpha coefficient indicated that the internal consistency for the questions, which concentrated on the hands (10 questions, α = 0.71) and feet (7 questions, α = 0.81), was suitable. Therefore, it was allowed to sum the individual scores of the questions. To our opinion, however, one should be cautious with the interpretation of the sum-scores. A higher score can not automatically be designated as a higher level of clinical neuropathy. The sum-scores ranged between 0 (no clinical neuropathy) and 9 (median: 2) for the hands and between 0 (no clinical neuropathy) and 18 (median: 3) for the feet. Because of the small study population, the data were transformed to a dichotomous scale (neuropathy yes or no) and no ordinal logistic regression analyses on the non-transformed data were performed.
The SRT test revealed that 29 patients (11/20 for cisplatin (55%), 18/24 for oxaliplatin (75%)) were classified as having an abnormal balance. For one oxaliplatin patient the SRT test could not be tested. The TG test was normal in 24 patients, whereas 15 (4/20 for cisplatin (20%), 11/22 for oxaliplatin (50%)) patients experienced difficulties to perform the test and 3 (2/20 for cisplatin (10%), 1/22 for oxaliplatin (4.5%)) were not able to perform it. For three oxaliplatin patients the TG could not be tested.
Vibration perception results
In a and b, the individual VT values of the patients for, respectively, the hands and feet are plotted versus the age of the patients. It has been shown that age is an important confounder of the relation between VT and neuropathy Citation[24]. The figures also show the mean VT values (+ 2 standard deviations (SD)) plotted versus age for a normal population consisting of 110 controls Citation[24]. The VT-cut-off of 2 SD has been used to classify patients as having a normal or abnormal sensory nerve function Citation[16], Citation[34]. As can be derived from , hand-VT values which deviated ≥ 2 SD from the mean, were observed in 15 patients (8/20 for cisplatin (40%), 7/25 for oxaliplatin (28%)). For the feet-VT, this value was 18 (7/20 for cisplatin (35%), 11/25 for oxaliplatin (44%)).
Figure 2. Hands and feet VT values for cisplatin (▵) and oxaliplatin (○) treated patients and the mean values (♦) (+2 SD (▪)) for 110 control patients Citation[23] plotted against the age
![Figure 2. Hands and feet VT values for cisplatin (▵) and oxaliplatin (○) treated patients and the mean values (♦) (+2 SD (▪)) for 110 control patients Citation[23] plotted against the age](/cms/asset/b723a4b5-10e1-4a42-baa6-a347c86da144/ionc_a_380830_f0002_b.gif)
Comparison of questionnaire scores with vibration perception results
The classification of patients on the questionnaire data (neuropathy or no neuropathy) showed that a higher number of patients actually experienced neuropathy than the number that was designated as neuropathic using the VT test. Sensitivity and specificity values of the dichotomous VT scale with a cut-off of 2 SD were calculated considering the dichotomous questionnaire score as the correct values (). Obviously, not all patients were classified correctly. Therefore, it was investigated whether the application of the VT test could be improved by using other VT cut-off values. The sensitivity and specificity of several cut-off values were investigated by the composition of ROC curves. The dichotomous questionnaire scores were used as state variables. The natural logarithms (ln) of the VT values which were normalised for age were used as test variables. Normalised VT values were obtained by dividing the VT values of the patients by the mean VT values assessed in controls with the same age as the patient Citation[24]. The natural logarithms (ln) of these values ranged from −0.342 to 4.20 for the hands and from −1.39 to 4.30 for the feet. Optimal sensitivity and specificity values were obtained with an ln VT value of 0.860 for the hands and 1.35 for the feet. These values corresponded to cut-offs of 1.4 and 1.7 SD, respectively for the hands and feet. Using these cut-offs, sensitivity and specificity values were respectively 77 and 63% for the hands and 72 and 83% for the feet (). These values were significantly better than those obtained with a cut-off value of 2 SD. Using these cut-off values elevated VT values were observed in 27 patients for the hands and 25 patients for the feet.
Table IIIa. Sensitivity and specificity for a VT-cut-off value of 2 SD
Table IIIb. Sensitivity and specificity for VT-cut-off values of 1.4 (hands) and 1.7 (feet) SD
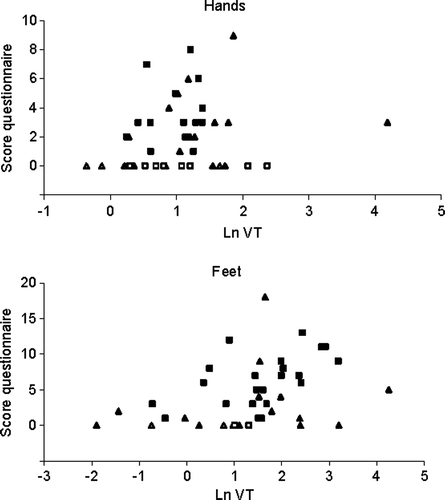
The use of a dichotomous VT scale solely provides information regarding the presence or absence of neuropathy and quantitative information on further grading is not obtained. Hence, in addition to the generally used dichotomous scale, the ln VT values were also used to describe the neuropathy based on a continuous scale. To assess whether the ln VT values could predict the subjective experience of the patients, the sum-scores of the questionnaire were plotted versus the ln VT values (). It was observed that, in general, raised questionnaire scores were coupled to high ln VT scores for the hands as well as for the feet.
Comparison of neurological tests with questionnaire scores and vibration perception results
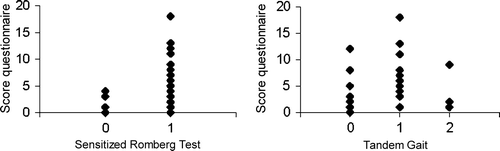
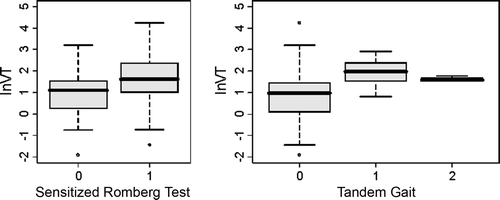
To assess whether the questionnaire scores were associated to the neurological tests, the sum-scores of the questionnaire results for the feet were plotted versus the SRT and TG scores (). The association between the ln VT values for the feet and SRT and TG scores was evaluated by boxplots (). It was observed that, in general, high ln VT values were coupled to abnormal SRT and TG scores. This relationship, however, was not observed for the questionnaire and SRT and TG scores. Therefore, VT measurements were used for further data analyses.
Effects of determinants on ln VT
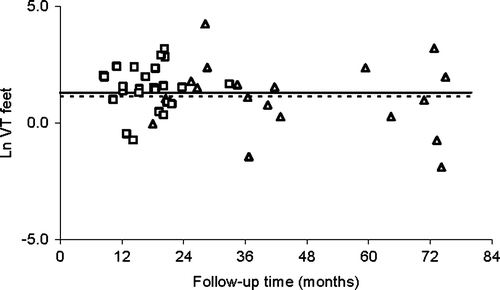
Possible determinants that could affect the sensory nerve function were evaluated using the VT measurements. Ln VT values for the hands and feet were plotted against the follow-up time in and , respectively. The ln VT values for the hands showed a small decline with follow-up time following a first order decay with a half-life (t1/2) of 6.8 (± 3.1) years. The observed recovery t1/2 was similar for cisplatin and oxaliplatin and was related to age, in which a twofold increase in age resulted in a three-fold longer recovery t1/2 (p = 0.02). For the feet, no decline of the ln VT value with time was observed.
Figure 5a. Ln VT values for the hands for cisplatin (▴) and oxaliplatin (▪) treated patients versus time and a median patients treated with 300 mg/m2 cisplatin (––––), with 600 mg/m2 cisplatin combined with STS (-----), and with oxaliplatin 878 mg/m2 (-------)
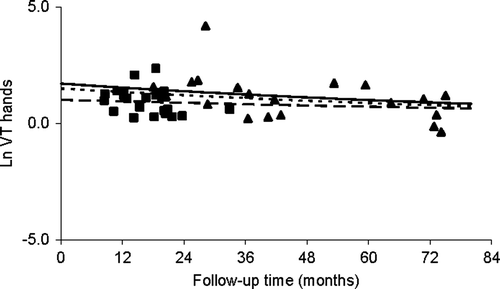
Figure 5b. Ln VT values for the feet for cisplatin (▵) and oxaliplatin (□) treated patients versus time and a median patients treated with 300 mg/m2 cisplatin (–––), with 600 mg/m2 cisplatin combined with STS (-----), and with oxaliplatin 878 mg/m2 (-------)
Plotting of the dichotomous results of the VT test versus the follow-up time did not result in any additional information. The continuous scale for neuropathy assessment obtained from the ln VT measurement was considered more informative than the dichotomous classification and was, therefore, chosen for further data analysis.
The ln VT values for cisplatin were higher than for oxaliplatin. For cisplatin, ln VT values were proportional to the dose. On the contrary, no dose dependency was observed for oxaliplatin. STS co-administration with intra-arterial cisplatin administration led to 56% reduction of ln VT for cisplatin (p = 0.002) both in the hands and feet. No effect of concomitant infusion of calcium and magnesium on VT measurements for oxaliplatin treated patients could be demonstrated because 24 patients received this concomitant infusion and only one patient did not.
Curves of modelled ln VT values against the follow-up time for a median aged patient who received 600 mg/m2 cisplatin with STS, a patient who received 300 mg/m2 cisplatin, and a patient who received 878 mg/m2 oxaliplatin are depicted in .
The observed plasma Pt levels at the time of follow-up and GST allele frequencies are shown in Table II. It was not considered plausible that Pt levels at the time of follow-up were related to nerve function, because plasma Pt levels decrease with time Citation[32], whereas Pt levels in ganglia remain constant over time Citation[13]. Therefore, Pt levels were extrapolated to the time of treatment using the elimination half-life Citation[32]. No relationship between plasma Pt levels and ln VT values was observed. Furthermore, an association between GSTT1, GSTM1, and GSTP1 genotypes and ln VT values could not be established. The effect of the renal function on ln VT values was evaluated using the MDRD-GFR. Median GRF values before start of the Pt chemotherapy were 78 and 65 mL/min/1.73m2 for cisplatin and oxaliplatin, respectively (Table II). At the time of the current study, GFR values of cisplatin patients were significantly decreased to 55 mL/min/1.73m2 (p < 0.001), whereas oxaliplatin GFR values remained constant (61 mL/min/1.73m2). No association was observed between GFR and ln VT values. Furthermore, alcohol use, diabetes mellitus, or co-medication did not seem to affect ln VT values.
Discussion
Cisplatin and oxaliplatin are frequently used chemotherapeutic agents. Their use, however, is hampered by a peripheral sensory neuropathy, which can, even years after cessation of treatment, seriously affect the quality of life.
The aims of the current explorative study were to assess persistent neuropathy in 45 patients more than eight months and up to 75 months after treatment with cisplatin or oxaliplatin and to determine the most adequate method to evaluate neuropathy. The assessment of neuropathy was performed using a questionnaire and by neurological tests. Besides, neuropathy was assessed quantitatively by VT measurements.
Comparison of the questionnaire results and VT values showed that the generally used 2 SD cut-off value for the VT measurement may not lead to adequate classification of neuropathy. Therefore, classification was improved by using different cut-off values. However, by classifying the dichotomous VT measurements, quantitative information regarding the nerve function was lost. Hence, the ln VT values were used to describe the neuropathy based on a continuous scale. It was observed that, in general, raised questionnaire sum-scores were coupled to high ln VT scores for the hands as well as for the feet. When questionnaire sum-scores and ln VT values were plotted versus the scores of the neurological tests, ln VT values were more obviously associated to the neurological tests than the questionnaire sum-scores. Because quantitative measurements may provide more precise and objective measures of neuropathy, the VT determination was used to perform further data analyses.
Investigation of the effects of determinants on ln VT values revealed that the ln VT values for the hands declined with follow-up time. The recovery t1/2 of 6.8 (± 3.1) years, however, suggests that for both cisplatin and oxaliplatin, the reversibility is slow and incomplete. The observation that a twofold increase in age resulted in a three-fold longer recovery t1/2, suggests that the effect of age on VT values in our population was larger than the effect observed in the population of Goldberg et al. Citation[24]. Probably, the sensory nerve function of patients treated with Pt agents is more susceptible to age than the nerve function of normal individuals.
The observations that ln VT values for the feet did not decline with time suggests that neuropathy in the feet is an even more important issue than neuropathy in the hands. This suggestion was amplified by the results of the questionnaire score, which showed that neuropathy in the feet was experienced by 32 patients, whereas neuropathy in the hands was experienced by 26 patients. This was in accordance with an investigation performed by Land et al. who mentioned that for oxaliplatin, 18 months after the start of treatment, neuropathy was primarily present in the feet Citation[18]. The apparent larger effect of Pt agents on the feet compared to the hands is in accordance with the observation that other determinants such as age also affect the sensory nerve function in the feet more than in the hands Citation[24].
The ln VT values for cisplatin were proportional to the cumulative dose. This association was already extensively described in previous studies Citation[8], Citation[35], Citation[36]. In contrast, no association between dose and ln VT for oxaliplatin was observed. This might be a consequence of the limited dose range studied in these patients. In contrast to our observations, other studies mentioned the presence of a dose dependency for persistent oxaliplatin induced neuropathy Citation[6], Citation[11].
The ln VT values were not related to the plasma Pt levels at the time of chemotherapy. Plasma Pt levels at the time of chemotherapy were estimated by extrapolating the plasma Pt levels at follow-up using the estimated elimination t1/2. Because the elimination t1/2 was estimated using only one data point per patient, this could have resulted in an inaccurate estimation of Pt levels at the time of chemotherapy. Therefore, to evaluate the relationship between plasma Pt levels and ln VT, more data points per patient are indispensable.
The association between STS, calcium/magnesium co-administration, and ln VT values was also evaluated. STS co-administration with intra-arterial cisplatin administration led to 56% reduction in ln VT for cisplatin. By binding to cisplatin, STS can inactivate cisplatin. This might lead to a reduction of Pt load in tissue and thereby a reduction of toxicity. Because 24 of the 25 evaluated oxaliplatin patients received concomitant calcium/magnesium infusions, the group of patients not treated with calcium and magnesium was too small to be able to observe an effect on the ln VT values. However, the possible effect of calcium and magnesium should be taken into account in future studies. There are no studies available describing the difference between i.a. administered equal doses of cisplatin with or without co-administration of STS. Therefore, the contribution of intra-arterial administration to the reduction of ln VT levels is not clear.
It would be reasonable that the renal function at the time of chemotherapy affected the severity of neuropathy. A better renal function could imply a faster initial elimination of Pt, leading to lower tissue levels and thus less toxicity. This association, however, was not observed in the current population. This could be due to the small sample size and a limited variation in GFR.
Genetic factors, that potentially influence the pharmacokinetics of an anticancer drug and thereby the development of drug toxicity in patients could be relevant determinants in this study. The detoxifying GST enzymes are thought to participate collectively in the intracellular metabolism and detoxification Citation[37], Citation[38]. Recent investigations showed that patients homozygous or heterozygous for the GSTP1 105Val allele were less susceptible for developing severe oxaliplatin-induced neurotoxicity Citation[39]. The presence of both alleles of 105Val-GSTP1 offered protection against cisplatin-induced hearing impairment Citation[40]. In addition, Oldenburg and colleagues showed that the presence of both GSTP1-G alleles and/or absence of functional GSTM1 protected against Pt induced ototoxicity and neuropathy Citation[41]. Although it might be suspected that mutations in the GSTs genes could affect sensory neuropathy, we did not observe an association between ln VT and any of the three (GSTM1, GSTT1 and GSTP1) genes investigated, which is probably due to the small sample size of the current population.
To summarise, the effect of possible determinants on neuropathy could be evaluated using continuous VT values, which provides the most extensive information about the severity of neuropathy. Our data suggest that neuropathy in the hands is slowly reversible, whereas no reversibility of neuropathy in the feet could be demonstrated during follow-up in the current study. Furthermore, for cisplatin, the severity of neuropathy was associated with cumulative dose and STS use. Oxaliplatin induced neuropathy did not appear to be related to the dose. No relationship with renal function, GST genotypes, diabetes mellitus, alcohol use, or co-medications could be demonstrated. This study was performed as an explorative study and the issues discussed need to be investigated further.
Acknowledgements
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Mathé G, Kidani Y, Triana K, Brienza S, Ribaud P, Goldschmidt E, et al. A phase I trial of trans-1-diaminocyclohexane oxalato-platinum (l-OHP). Biomed Pharmacother 1986; 40: 372–6
- Pendyala L, Creaven PJ. In vitro cytotoxicity, protein binding, red blood cell partitioning, and biotransformation of oxaliplatin. Cancer Res 1993; 53: 5970–6
- Schmidt W, Chaney SG. Role of carrier ligand in platinum resistance of human carcinoma cell lines. Cancer Res 1993; 53: 799–805
- Chaudhary UB, Haldas JR. Long-term complications of chemotherapy for germ cell tumours. Drugs 2003; 63: 1565–77
- Alberts DS, Noel JK. Cisplatin-associated neurotoxicity: Can it be prevented?. Anticancer Drugs 1995; 6: 369–83
- Grothey A. Oxaliplatin-safety profile: Neurotoxicity. Semin Oncol 2003; 30(4Suppl 15)5–13
- Boogerd W, Bokkel Huinink WW, Dalesio O, Hoppenbrouwers WJ, van der Sande JJ. Cisplatin induced neuropathy: central, peripheral and autonomic nerve involvement. J Neurooncol 1990; 9: 255–63
- Siegal T, Haim N. Cisplatin-induced peripheral neuropathy. Frequent off-therapy deterioration, demyelinating syndromes, and muscle cramps. Cancer 1990; 66: 1117–23
- Taieb S, Trillet-Lenoir V, Rambaud L, Descos L, Freyer G. Lhermitte sign and urinary retention: Atypical presentation of oxaliplatin neurotoxicity in four patients. Cancer 2002; 94: 2434–40
- LoMonaco M, Milone M, Batocchi AP, Padua L, Restuccia D, Tonali P. Cisplatin neuropathy: Clinical course and neurophysiological findings. J Neurol 1992; 239: 199–204
- Pietrangeli A, Leandri M, Terzoli E, Jandolo B, Garufi C. Persistence of high-dose oxaliplatin-induced neuropathy at long-term follow-up. Eur Neurol 2006; 56: 13–6
- McWhinney SR, Goldberg RM, McLeod HL. Platinum neurotoxicity pharmacogenetics. Mol Cancer Ther 2009; 8: 10–6
- Gregg RW, Molepo JM, Monpetit VJ, Mikael NZ, Redmond D, Gadia M, et al. Cisplatin neurotoxicity: The relationship between dosage, time, and platinum concentration in neurologic tissues, and morphologic evidence of toxicity. J Clin Oncol 1992; 10: 795–803
- Heydorn K, Rietz B, Krarup-Hansen A. Distribution of platinum in patients treated with cisplatin determined by radiochemical neutron activation analysis. J Trace Elem Exp Med 1998; 11: 37–43
- McKeage MJ, Hsu T, Screnci D, Haddad G, Baguley BC. Nucleolar damage correlates with neurotoxicity induced by different platinum drugs. Br J Cancer 2001; 85: 1219–25
- Postma TJ, Hoekman K, van Riel JM, Heimans JJ, Vermorken JB. Peripheral neuropathy due to biweekly paclitaxel, epirubicin and cisplatin in patients with advanced ovarian cancer. J Neurooncol 1999; 45: 241–6
- Leonard GD, Wright MA, Quinn MG, Fioravanti S, Harold N, Schuler B, et al. Survey of oxaliplatin-associated neurotoxicity using an interview-based questionnaire in patients with metastatic colorectal cancer. BMC Cancer 2005; 5: 116
- Land SR, Kopec JA, Cecchini RS, Ganz PA, Wieand HS, Colangelo LH, et al. Neurotoxicity from oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: NSABP C-07. J Clin Oncol 2007; 25: 2205–11
- van der Hoop RG, Vecht CJ, van der Burg ME, Elderson A, Boogerd W, Heimans JJ, et al. Prevention of cisplatin neurotoxicity with an ACTH(4-9) analogue in patients with ovarian cancer. N Engl J Med 1990; 322: 89–94
- Petersen PM, Hansen SW. The course of long-term toxicity in patients treated with cisplatin-based chemotherapy for non-seminomatous germ-cell cancer. Ann Oncol 1999; 10: 1475–83
- Postma TJ, Aaronson NK, Heimans JJ, Muller MJ, Hildebrand JG, Delattre JY, et al. The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: The QLQ-CIPN20. Eur J Cancer 2005; 41: 1135–9
- Gerr F, Letz R. Vibrotactile threshold testing in occupational health: A review of current issues and limitations. Environ Res 1993; 60: 145–59
- Chong PS, Cros DP. Technology literature review: Quantitative sensory testing. Muscle Nerve 2004; 29: 734–47
- Goldberg JM, Lindblom U. Standardised method of determining vibratory perception thresholds for diagnosis and screening in neurological investigation. J Neurol Neurosurg Psych 1979; 42: 793–803
- Chuang HY, Schwartz J, Tsai SY, Lee ML, Wang JD, Hu H. Vibration perception thresholds in workers with long term exposure to lead. Occup Environ Med 2000; 57: 588–94
- Boom R, Sol CJ, Salimans MM, Jansen CL, Wertheim-van Dillen PM, van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol 1990; 28: 495–503
- Pemble S, Schroeder KR, Spencer SR, Meyer DJ, Hallier E, Bolt HM, et al. Human glutathione S-transferase theta (GSTT1): cDNA cloning and the characterization of a genetic polymorphism. Biochem J 1994; 300(Pt 1)271–6
- Ali-Osman F, Akande O, Antoun G, Mao JX, Buolamwini J. Molecular cloning, characterization, and expression in Escherichia coli of full-length cDNAs of three human glutathione S-transferase Pi gene variants. Evidence for differential catalytic activity of the encoded proteins. J Biol Chem 1997; 272: 10004–12
- Sreelekha TT, Ramadas K, Pandey M, Thomas G, Nalinakumari KR, Pillai MR. Genetic polymorphism of CYP1A1, GSTM1 and GSTT1 genes in Indian oral cancer. Oral Oncol 2001; 37: 593–8
- Jeronimo C, Varzim G, Henrique R, Oliveira J, Bento MJ, Silva C, et al. I105V polymorphism and promoter methylation of the GSTP1 gene in prostate adenocarcinoma. Cancer Epidemiol Biomarkers Prev 2002; 11: 445–50
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130: 461–70
- Brouwers, EEM, Huitema, ADR, Beijnen, JH, Schellens, JHM. Long-term platinum retention after treatment with cisplatin and oxaliplatin. BMC Clin Pharmacol 2008.
- Bland JM, Altman DG. Statistics note: Cronbach's alpha. Br Med J 1997; 314: 572
- Somedic, AB. Stockholm Sweden. Manual for vibrameter Type III. 1985.
- van der Hoop RG, van der Burg ME, Bokkel Huinink WW, van Houwelingen C, Neijt JP. Incidence of neuropathy in 395 patients with ovarian cancer treated with or without cisplatin. Cancer 1990; 66: 1697–702
- Bokemeyer C, Berger CC, Kuczyk MA, Schmoll HJ. Evaluation of long-term toxicity after chemotherapy for testicular cancer. J Clin Oncol 1996; 14: 2923–32
- Tew KD. Glutathione-associated enzymes in anticancer drug resistance. Cancer Res 1994; 54: 4313–20
- Zhang K, Chew M, Yang EB, Wong KP, Mack P. Modulation of cisplatin cytotoxicity and cisplatin-induced DNA cross-links in HepG2 cells by regulation of glutathione-related mechanisms. Mol Pharmacol 2001; 59: 837–43
- Lecomte T, Landi B, Beaune P, Laurent-Puig P, Loriot MA. Glutathione S-transferase P1 polymorphism (Ile105Val) predicts cumulative neuropathy in patients receiving oxaliplatin-based chemotherapy. Clin Cancer Res 2006; 12: 3050–6
- Oldenburg J, Kraggerud SM, Cvancarova M, Lothe RA, Fossa SD. Cisplatin-induced long-term hearing impairment is associated with specific glutathione s-transferase genotypes in testicular cancer survivors. J Clin Oncol 2007; 25: 708–14
- Oldenburg J, Kraggerud SM, Brydoy M, Cvancarova M, Lothe RA, Fossa SD. Association between long-term neuro-toxicities in testicular cancer survivors and polymorphisms in glutathione-s-transferase-P1 and -M1, a retrospective cross sectional study. J Transl Med 2007; 5: 70