Abstract
Background. Hypoxia-inducible factor (HIF)-2α is upregulated in hypoxia or by inactivation of the von Hippel-Lindau (VHL) tumour suppressor gene. In a number of malignancies, increased HIF-2α expression may indicate worse prognosis. The aim of this study was to evaluate the prognostic information of HIF-2α mRNA expression in renal cell carcinoma (RCC). Material and methods. HIF-2α mRNA was quantified by real time polymerase chain reaction (rt-PCR) in tumour tissue samples from 202 patients. Samples from 50 corresponding kidney cortex tissue were analysed as controls. mRNA levels were evaluated in relation to tumour cell type, TNM stage, nuclear grade and disease specific survival. Results. The levels of HIF-2α mRNA were significantly higher in 168 clear cell (c)RCC than in 23 papillary (p)RCC (p < 0.001) or 11 chromophobe (ch)RCC (p < 0.006). Among cRCC there was an inverse correlation between HIF-2α mRNA levels and TNM stage I and II-IV tumours (p=0.01), and nuclear grade (p = 0.006). After a median follow-up time of 99 months (range 34–247), 106 patients had died of RCC. No correlation of HIF-2α mRNA to survival was observed. A multivariate analysis of prognostic factors in cRCC showed that TNM stage alone was an independent predictor of prognosis; HIF-2α mRNA levels did not add further prognostic information. Discussion. The results demonstrated that HIF-2α mRNA levels were higher in cRCC compared to pRCC and chRCC. Furthermore, HIF-2α mRNA levels were inversely related to TNM stage and nuclear grade in cRCC.
Tumour cells must be able to rapidly respond to sudden changes in oxygen levels in order to survive in a hypoxic environment. A number of systemic and cellular responses have been developed to enable organisms to respond to hypoxia, including glycolysis, angiogenesis, vasodilation, and erythropoesis Citation[1]. Micro-environmental hypoxia in tumours induces via hypoxia-inducible factor (HIF) an upregulation of genes associated with angiogenesis, glycolysis, pH adaptation, erythropoesis, glucose transport, and apoptosis Citation[2], Citation[3]. Interference with this pathway has been proposed as a potential cancer therapy.
HIF is a basic helix-loop-helix heterodimeric transcription factor consisting of a constitutively expressed β subunit and one of three oxygen-sensitive α subunits (HIF-1α, HIF-2α [EPAS1] or HIF-3α) Citation3–5. The HIF-α subunits are regulated in similar ways by activation of the phosphatidylinositol 3-kinase (PI3K) and mTOR, or by the extracellular signal-regulated kinase (ERK) Citation[2], but occasionally have different biological functions Citation[3], Citation[5]. The HIF-α subunits are normally unstable in the presence of oxygen, but are stabilized in hypoxia. It has been suggested that HIF-2α over-expression is important in the development of renal cell carcinoma (RCC), and that it may act as an oncogene Citation[6], Citation[7]. HIF-2α has been reported to be expressed in cRCC, but absent or present in low levels, in other RCC types and in normal kidney cortex Citation[8]. High HIF-1α levels are associated with decreased mortality among patients with cRCC Citation[9]. In other malignancies, like cancer of the head and neck region, high HIF-2α expression may be related to poor outcome Citation[10].
In cancer, the HIF system is activated by micro-environmental hypoxia and by other pathways. Activation of the HIF pathway is observed in tumours associated with inactivation of the von Hippel-Lindau (VHL) tumour suppressor gene Citation[2], Citation[3]. VHL mutations are associated with sporadic cRCC in 50–70% of the patients Citation[11]. VHL-defective RCC cells seem to show a bias toward HIF-2α rather than HIF-1α expression Citation[12], Citation[13]. Presence of VHL-mutation, as well as loss of pVHL expression among patients with cRCC and early-stage disease, is associated with improved survival compared with patients without this defect Citation[14], Citation[15].
The aim of this study was to evaluate the role of HIF-2α mRNA and its potential prognostic information in RCC.
Material and methods
Patients
This retrospective study included 202 patients (117 men, 85 women) with histopathologically verified RCC, admitted to and nephrectomised at the Department of Urology, Umeå University Hospital, Sweden, between 1985 and 2003 (). Patient median age was 67 (range, 34–85) years. The patients underwent a physical examination, chest radiography, and computer tomography (CT) of the abdomen. When vena cava tumour thrombus invasion was suspected, cavography or magnetic resonance imaging (MRI) was performed. Patients with skeletal-associated pain or elevated serum alkaline phosphatase were assessed with bone scintigraphy. The patients were followed-up according to a program including regular clinical and radiological examinations. The median follow-up time was 99 (range, 34–247) months. Only a few patients with residual disease after nephrectomy or recurrent disease were treated with tamoxifen or interferon. At the end of follow-up, 106 patients had died of RCC, 40 of other diseases and 56 were alive. The cause of death was based on death certificates and patient charts.
Table I. Clinical characteristics of the patients with RCC.
All tumour samples were obtained with permission from the patients and during the later years with informed and signed consent. The study was approved by the ethics committee of Umeå University.
Tumour cell type, stage, grade, size
The RCC types were assessed according to the Heidelberg classification system Citation[16]. The patients presented with clear cell (n = 168), papillary (n = 23), or chromophobe (n = 11) RCC. From 50 patients (39 cRCC, 8 pRCC and 3 chRCC) biopsies from corresponding kidney cortex were taken as a control. Tumour staging was performed according to the TNM classification system 2002 Citation[17], and nuclear grading according to Fuhrman et al. Citation[18]. Tumour size was measured at the maximum diameter on the surgical specimen or by CT. The median tumour diameter was 8.1 (range, 2.0–17.0) cm.
Tissue samples
Tumour and kidney cortex tissue samples were obtained from surgical specimen and immediately frozen in liquid nitrogen and stored at −80°C until analysis.
RNA and cDNA preparation
Total RNA was isolated from the frozen specimens using the TRIzol method (Invitrogen, Stockholm, Sweden) and was quantified spectrophotometrically at 260 nm (Lamda 2 UV/VIS, Perkin Elmer, Stockholm, Sweden). Electrophoresis was then run and RNA quality was verified by ethidium bromide staining of 18S and 28S rRNA, and/or with the Agilent 2100 Bioanalyzer (Agilent Technologies, Germany) on selected samples. Complementary (c)DNA was made from 250 ng total RNA (all samples in duplicates) by Superscript II reverse transcriptase (Invitrogen, Stockholm, Sweden) in 20 L reaction volume according to the manufacturer's instructions by PCR.
Quantification of HIF-2α mRNA
Quantification of HIF-2α mRNA was done by real time PCR using LightCycler FastStart DNA Master SYBR Green I technology (Roche Applied Science) and a LightCycler instrument (Roche). PCR was performed in 20 L reaction volume, containing cDNA corresponding to 25 ng RNA, 3 mM MgCl2, 2 L DNA Master SYBR Green I, and 0.5 M of each primer (Hif-2α Left: 5′- TTG ATG TGG AAA CGG ATG AA–3′ Right: 5′- GGA ACC TGC TCT TGC TGT TC–3′). The PCR reaction was initiated with a 10-min activation step at 95°C, followed by 45 cycles of 95°C denaturation for 15 s, 62°C primer annealing for 10 s, and 72°C extension for 10 s. A melting curve at 98°C for PCR product identification was done. Negative controls were run to confirm that the samples were not cross contaminated. The relative mRNA values of HIF-2α in the samples were calculated from a standard curve of five-fold serial dilution of reversed transcribed total RNA from a reference sample and one control. The target values are presented as relative values in comparison to the reference sample.
Statistical methods
Statistical analysis was performed using Mann-Whitney U and Kruskal-Wallis tests. Computer soft ware SPSS version 13.0 was used. Correlations between variables were tested according to the Spearman correlation test. Survival was assessed and illustrated using the Kaplan-Meier method, and survival time was compared using the log-rank test. Multivariate regression analysis was performed by the Cox proportional-hazards model. P-values of < 0.05 were considered statistically significant.
Results
HIF-2α mRNA expression
HIF-2α mRNA was present in all tissue samples analysed. mRNA levels in pRCC, but not in cRCC or chRCC tumours, was lower than in the corresponding kidney cortex tissue (p = 0.016, ). No difference in HIF-2α mRNA levels between kidney cortex tissues sampled from different tumour cell types was observed. The cRCC tumours showed higher HIF-2α mRNA levels compared to pRCC (p < 0.001) or chRCC (p = 0.006), while there was no significant difference between pRCC and chRCC (). No correlation of HIF-2α mRNA levels to age, gender or storage time was observed.
Table II. Relative HIF-2α mRNA levels in kidney cortex and tumour tissue from corresponding kidney in different renal cell carcinoma types.
Table III. Relative HIF-2α mRNA levels comparing levels in tumour tissue from different renal cell carcinoma types.
Clinical parameters
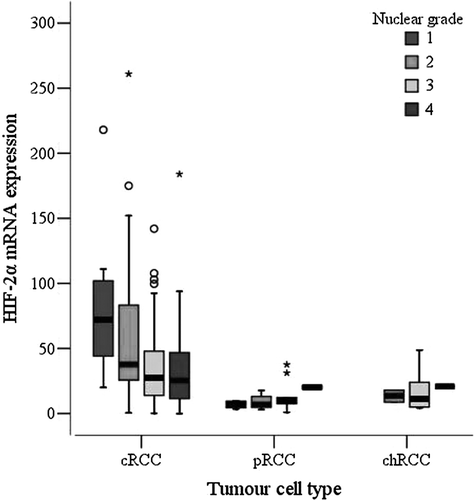
When cRCC were subdivided according to TNM stage, tumours with TNM stage I had significantly higher HIF-2α mRNA levels than stage II-IV tumours, as shown in (p = 0.01, Mann-Whitney test). In analogy with the observed correlation to TNM stage, there was a weak inverse correlation between HIF-2α mRNA levels and tumour size among patients with cRCC (r = -0.252; p = 0.001, Spearman correlation). This was not observed for the limited number of pRCC or chRCC. Furthermore, an inverse correlation between HIF-2α mRNA levels and nuclear grade in cRCC was observed (p = 0.006, Kruskal-Wallis test). Tumours with high HIF-2α mRNA levels had lower nuclear grades (). For pRCC and chRCC no relation between HIF-2α mRNA expression and nuclear grade was found.
Figure 1. Box-and-whisker plot of relative HIF-2α mRNA levels in relation to TNM stage in 168 cRCC, 23 pRCC, and 11 chRCC (cRCC: TNM I = 46, TNM II = 30, TNM III = 37, TNM IV = 55; pRCC: TNM I = 7, TNM II = 6, TNM III = 5, TNM IV = 5; chRCC: TNM I = 1, TNM II = 4, TNM III = 4, TNM IV = 2). Comparison of Hif-2α mRNA in TNM stage I versus TNM stage II-IV in cRCC (p = 0.01, Mann-Whitney test). Circles and asterisks represent outlying values, >1.5 and >3 box-lengths from the 75th percentile, respectively.
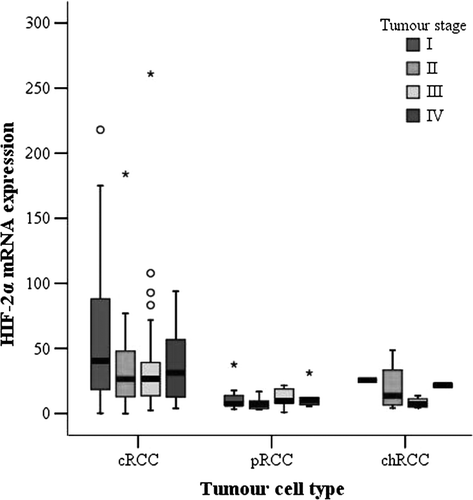
Figure 2. Box-and-whisker plot of relative HIF-2α mRNA levels in relation to nuclear grade (NG) in 168 cRCC, 23 pRCC, and 11 chRCC (cRCC: NG1 = 8, NG2 = 33, NG3 = 90, NG4 = 37; pRCC: NG1 = 4, NG2 = 8, NG3 = 9, NG4 = 2; chRCC: NG1 = 0, NG2 = 2, NG3 = 8, NG4 = 1). Trend for HIF-2α mRNA and nuclear grade 1-4 in cRCC (p = 0.006, Kruskal-Wallis test). Circles and asterisks represent outlying values.
Survival
To evaluate survival, patients were divided into two groups at the median HIF-2α mRNA (in cRCC: HIF-2αlow < 29.10 relative levels, HIF-2αhigh≥ 29.10 relative levels). No correlation between disease-specific survival and HIF-2α mRNA levels in any RCC type was seen. Median (range) survival time was 38 (0–247), 51 (3–236), and 70 (2–179) months for cRCC, pRCC, and chRCC, respectively.
Multivariate analysis
To analyse the prognostic information of HIF-2α mRNA in cRCC, a multivariate analysis using Cox proportional hazard model was performed. Age, gender, TNM stage, nuclear grade, HIF-2α mRNA levels and tumour specific survival were evaluated. After the final step analysis, as shown in , only TNM stage presented as an independent prognostic factor. HIF-2α mRNA levels added no prognostic information.
Table IV. Multivariate analysis of prognostic factors in 168 patients with clear cell renal cell carcinoma.
Discussion
In cancer, micro-environmental hypoxia is the main regulator of the HIF complex program of gene transcription. In the presence of hypoxia or VHL mutation, HIF-α stabilizes and accumulates, forms a heterodimer with HIF-β, and binds to hypoxic response elements Citation[2], Citation[3]. HIF-2α expression is related to tumour progression in head and neck, and kidney cancer Citation6–8, Citation[10].
In this study, we showed that HIF-2α mRNA was present in all tumours and kidney cortex tissue assessed, and that the levels were higher in cRCC than in pRCC and chRCC, which is in line with observations on mRNA and protein expression Citation[8], Citation[13]. Earlier reports show that HIF-2α was present in cRCC while being absent, or seen in low levels, in other RCC types or in kidney cortex. Still, both cRCC and chRCC expressed HIF-2α mRNA levels in kidney cortex that were not different from those assessed in corresponding tumour tissue. Despite the fact that kidney cortex from the tumour-bearing kidney may look histologically normal, this tissue could be fenotypically abnormal or exhibit altered gene expression profiles because of the proximity to the tumour Citation[19], Citation[20], a fact that could explain the discrepancy between different studies.
We observed an inverse correlation between HIF-2α mRNA levels and TNM stage, nuclear grade, and tumour size among patients with cRCC. Over-expression of HIF-2α has been reported to promote growth of RCC cells Citation[6], Citation[7]. Small interfering RNA-mediated down-regulation of HIF-2α itself suppresses tumour formation by VHL-defective RCC cells Citation[21], Citation[22].
Both hereditary and sporadic cRCC are commonly associated with VHL mutation which is found in 50–70% of sporadic cRCC Citation[11]. HIF-2α protein is up-regulated by hypoxia or VHL mutation, independently of each other Citation[13]. These findings suggest that VHL-associated RCC up-regulate HIF. However, other investigators have found that over-expression of a mutated HIF-1α gene does not block the tumour suppressor action of VHL in RCC cells Citation[6], raising the possibility that despite their similarities, and common up-regulation in many types of cancer, HIF-1α and HIF-2α may have non-equivalent effects in the pathogenesis of VHL-associated RCC. Raval et al. demonstrated a suppressive interaction between HIF-1α and HIF-2α, as well as an inhibiting effect of HIF-1α on tumour growth Citation[23]. HIF-2α, but not HIF-1α, regulates a constitutive VEGF expression in VHL-defective RCC cells, and the expression of a number of HIF targets is primarily driven by HIF-2α Citation[24].
Our results are in line with previous studies on RCC vascularity and hypoxia. Patients with cRCC with high immunohistochemical protein expression of CA IX had a more favourable prognosis, and microvascular density, assessed with endoglin or CD31, was inversely correlated to TNM stage and nuclear grade Citation25–27. The generally higher HIF-2α, CA IX, endoglin and CD31 expression in less aggressive cRCC could reflect the fact that other pathways apart from the HIF pathway might be activated in these tumours. It has been reported that presence of VHL-mutation as well as loss of pVHL expression among patients with cRCC and early-stage disease are associated with improved survival compared with patients without this defect Citation[14], Citation[15].
The real-time PCR technique allows quantification of small amounts of mRNA. Before real-time PCR is done, mRNA is copied to cDNA. It has been shown that the reverse transcriptase step contributes with most of the variation when quantifying mRNA Citation[28]. High accuracy is therefore needed in this part of the experiment, as well as during the processes of storing and handling of samples, especially the selection of samples from tumour and kidney cortex tissue is critical. Since SYBR green does not distinguish between one DNA and another, an important quality control is the melting curve. It visualizes that all samples have a similar melting temperature, i.e. that there is no contamination, mispriming, or primer-dimer artefacts.
This study was limited to the assessment of HIF-2α mRNA expression, and in cRCC tumours with low TNM stage and nuclear grade have the highest expression. From these results it could be hypothesized that patients with well differentiated cRCC would be those that could benefit most from targeted therapy. Further studies should aim at investigating HIF-2α protein levels.
In summary, we demonstrated that HIF-2α mRNA levels were higher in cRCC compared to other RCC types. Furthermore, in cRCC the HIF-2α mRNA levels were inversely related to TNM stage and nuclear grade. Despite these findings, HIF-2α mRNA levels did not affect survival. Evaluation of molecular markers such as HIF-2α might be important for reaching more precise prediction of individual tumour behaviour, and cancer treatment.
Acknowledgements
This study was supported by grants from Lions Research Foundation, the Department of Oncology, the Medical Faculty, Umeå University, and the Swedish Cancer Society. The authors are grateful to Björn Tavelin for excellent assistance with statistical analysis. Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Harris AL. Hypoxia – a key regulatory factor in tumour growth. Nat Rev Cancer 2002; 2: 38–47
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer 2003; 3: 721–32
- Haase VH. Hypoxia-inducible factors in the kidney. Am J Physiol Renal Physiol 2006; 291: F271–81
- Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix- loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA 1995; 92: 5510–4
- Bacon AL, Harris AL. Hypoxia-inducible factors and hypoxic cell death in tumour physiology. Ann Med 2004; 36: 530–9
- Maranchie JK, Vasselli JR, Riss J, Bonifacino JS, Linehan WM, Klausner RD. The contribution of VHL substrate binding and HIF1-alpha to the phenotype of VHL loss in renal cell carcinoma. Cancer Cell 2002; 1: 247–55
- Kondo K, Klco J, Nakamura E., Lechpammer M, Kaelin WG, Jr. Inhibition of HIF is necessary for tumor suppression by the von Hippel-Lindau protein. Cancer Cell 2002; 1: 237–46
- Turner KJ, Moore JW, Jones A, Taylor CF, Cuthbert-Heavens D, Han C, et al. Expression of hypoxia-inducible factors in human renal cell cancer: Relationship to angiogenesis and to the von Hippel-Lindau gene mutation. Cancer Res 2002; 62: 2957–61
- Lidgren A, Hedberg Y, Grankvist K, Rasmuson T, Vasko J, Ljungberg B. The expression of hypoxia-inducible factor 1alpha is a favorable independent prognostic factor in renal cell carcinoma. Clin Cancer Res 2005; 11: 1129–35
- Koukourakis MI, Bentzen SM, Giatromanolaki A, Wilson GD, Daley FM, Saunders MI, et al. Endogenous markers of two separate hypoxia response pathways (hypoxia inducible factor 2 alpha and carbonic anhydrase 9) are associated with radiotherapy failure in head and neck cancer patients recruited in the CHART randomized trial. J Clin Oncol 2006; 24: 727–35
- Kim WY, Kaelin WG. Role of VHL gene mutation in human cancer. J Clin Oncol 2004; 22: 4991–5004
- Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 1999; 399: 271–5
- Krieg M, Haas R, Brauch H, Acker T, Flamme I, Plate KH. Up-regulation of hypoxia-inducible factors HIF-1alpha and HIF-2alpha under normoxic conditions in renal carcinoma cells by von Hippel-Lindau tumor suppressor gene loss of function. Oncogene 2000; 19: 5435–43
- Parker AS, Cheville JC, Lohse CM, Igel T, Leibovich BC, Blute ML. Loss of expression of von Hippel-Lindau tumor suppressor protein associated with improved survival in patients with early-stage clear cell renal cell carcinoma. Urology 2005; 65: 1090–5
- Yao M, Yoshida M, Kishida T, Nakaigawa N, Baba M, Kobayashi K, et al. VHL tumor suppressor gene alterations associated with good prognosis in sporadic clear-cell renal carcinoma. J Natl Cancer Inst 2002; 94: 1569–75
- Kovacs G, Akhtar M, Beckwith BJ, Bugert P, Cooper CS, Delahunt B, et al. The Heidelberg classification of renal cell tumours. J Pathol 1997; 183: 131–3
- Sobin, L, Wittekind, CH, International Union Against Cancer (UICC) TNM Classification of malignant tumours. 6th ed. 2002. p 193–195.
- Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol 1982; 6: 655–63
- Deng G, Lu Y, Zlotnikov G, Thor AD, Smith HS. Loss of heterozygosity in normal tissue adjacent to breast carcinomas. Science 1996; 274: 2057–9
- Mocellin S, Rossi CR, Marincola FM. Quantitative real-time PCR in cancer research. Arch Immunol Ther Exp (Warsz) 2003; 51: 301–13
- Kondo K, Kim WY, Lechpammer M, Kaelin WG, Jr. Inhibition of HIF2alpha is sufficient to suppress pVHL-defective tumor growth. PLoS Biol 2003; 1: E83
- Sowter HM, Raval RR, Moore JW, Ratcliffe PJ, Harris AL. Predominant role of hypoxia-inducible transcription factor (Hif)-1alpha versus Hif-2alpha in regulation of the transcriptional response to hypoxia. Cancer Res 2003; 63: 6130–4
- Raval RR, Lau KW, Tran MG, Sowter HM, Mandriota SJ, Li JL, et al. Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau- associated renal cell carcinoma. Mol Cell Biol 2005; 25: 5675–86
- Carroll VA, Ashcroft M. Role of hypoxia-inducible factor (HIF)-1alpha versus HIF- 2alpha in the regulation of HIF target genes in response to hypoxia, insulin-like growth factor-I, or loss of von Hippel-Lindau function: Implications for targeting the HIF pathway. Cancer Res 2006; 66: 6264–70
- Sandlund J, Hedberg Y, Bergh A, Grankvist K, Ljungberg B, Rasmuson T. Endoglin (CD105) expression in human renal cell carcinoma. BJU Int 2006; 97: 706–10
- Sandlund J, Hedberg Y, Bergh A, Grankvist K, Ljungberg B, Rasmuson T. Evaluation of CD31 (PECAM-1) expression using tissue microarray in patients with renal cell carcinoma. Tumour Biol 2007; 28: 158–64
- Sandlund J, Oosterwijk E, Grankvist K, Oosterwijk-Wakka J, Ljungberg B, Rasmuson T. Prognostic impact of carbonic anhydrase IX expression in human renal cell carcinoma. BJU Int 2007; 100: 556–60
- Kubista M, Andrade JM, Bengtsson M, Forootan A, Jonak J, Lind K, et al. The real-time polymerase chain reaction. Mol Aspects Med 2006; 27: 95–125