Abstract
Background. The Swedish Insurance Company for Patient Injuries asked the two authors of this report to identify the Swedish women with cancer of the breast who had been injured by radiotherapy with a hypofractionated schedule. The purpose was to provide a basis on which the Company could decide if indemnification could be given. Material and methods. We define hypo-fractionation as any fraction dose exceeding 2.0 gray (Gy) per day. We set the lower limit for the “late effect” at 53.0 Gy with 2 Gy/fraction. All departments of radiotherapy in Sweden were asked to identify women who had developed brachial plexus neuropathy (BPN). Their medical records were obtained. The clinical picture of their injuries was recorded, and the absorbed dose was calculated or reconstructed. All doses, no matter in what way they were expressed, were recalculated to “late effect”, presented in EQD2Gy (Equalized Total Dose in 2 Gy/fraction). The latency period from therapy to onset of symptoms was also noted. Results. A variety of treatment techniques was used, fractions ranging in size from 2.5 to 6.0 Gy. Absorbed doses up to a Biologically Equivalent Dose (BED) 146 EQD2Gy in late effects were recorded (6 Gy×13). More than 95% of the injured women had a combination of stiff shoulder, paralysis, pain, oedema and atrophy of the muscles to the arm and/or hand. Latency from end of radiotherapy to onset of symptoms could be as long as 30 years. Discussion. Hypofractionated radiotherapy has injured severely numerous patients. The lesions have become a medico-legal issue in some countries. The life of many of these women has been ruined: physically, mentally, socially and economically. Conclusion. Hypofractionated radiotherapy can cause injuries if the target volume is not exact, or the total dose is not adjusted to a tolerable level as compared to conventional treatments employing 2 Gy/day fractions.
Introduction
Historical backgroundFootnote1
In the early 1960s, so called “hypofractionation” was introduced and used in several departments of radiotherapy and was maintained in some departments through the mid-1980s. The term “hypofractionation” has been given several definitions over the years. Literally, the term means “fewer treatments” (than the 5 or 6 fractions a week conventionally used).
There were two main reasons for employing hypofractionation. First, when mega-volt machines were introduced, even deep-seated tumors could be treated, which resulted in an increased demand for radiotherapy. This was not met with a corresponding increase in the number of treatment units, creating a shortage of treatment time. With fewer fractions per patient, more patients could be treated over time. Gilbert Fletcher – the legendary former professor of radiotherapy at M. D. Anderson Cancer Centre and one of the pioneering experts in radiotherapy – coined the phrase:
“Because the greatest part of the time used to treat a patient is spent on the set-up, the actual treatment time being only a fraction of the total time, it was logical to use fewer large fractions to decrease machine time.” Citation[1].
As time passed, it became evident that hypofractionated treatment schedules might injure patients. In some countries, the matter became a medico-legal issue. The present two authors have been asked by the Swedish Insurance Company for Patient Injuries to investigate the situation in Sweden. Thus, the purpose was not to make a scientific investigation but rather to provide a basis for the company on which it could decide if these injured patients could be given economic compensation.
Basic radiation parameters
“Conventional” fractionation may be defined as 2.0 gray (Gy)/day 5 times per week distributed over several weeks up to a total dose, which can vary from one type of tumour to another. With an administered total dose of 50.0 Gy, this fractionation will result in an “early effect” identical to the “late effect” of 50.0 Gy. There is no solid scientific rationale for the standard dose of 2.0 Gy; it is based purely on empirics and convenience with considerable experience of reasonable results. There is no criticism in this statement. As Jack Fowler put it:
“If therapists had waited for a fully scientific basis for treating the first patient, radiotherapy would not have started yet”. Citation[2]
Over the years, an almost perplexing number of fractionation schemes have been suggested. James D. Cox, the present Director of the Department of Radiotherapy at the M. D. Anderson Hospital in Texas, USA, summarized in 1985 the complexity:
“The possible combinations in clinical radiation therapy of daily dose, total dose and interval from the first treatment to the last, are, in principle, infinite, and, in practice, finite but too numerous to count” Citation[9].
Material and methods
Radiotheraputical aspects
At the start of this investigation, we addressed 14 internationally well-known radiation experts and asked them to answer two questions: 7 British, 6 American and 1 Australian (names withheld). We received ten answers to our first question:
“How do you define the term “hypofractionation”?”
The second question and the answers to it will be presented below.
One expert answered “Anything above 1.8–2.0 Gy/fraction”, one gave “above 2.0 and less than 5 fractions/week, two gave the answer “above 2.5 Gy/fraction” and the remaining six all gave the same answer: “anything above 2.0 Gy/fraction”. We joined those favouring “anything above 2.0 Gy/fraction”. The diverging opinions on the definition among the experts bring to mind the statement by Fletcher:
“There is a lack of consensus about every issue in radiotherapy including what are significant complications” Citation[10].
For our investigation of the damage caused by hypofractionation we had to set an upper acceptable total dose for the “late effect”. We arrived at a dose of 53.0 Gy (EQD2Gy) from the following reasoning: 48.0 Gy is the intermediate between the two most common upper dose limits to the axilla in Sweden: 46.0 and 50.0 Gy, respectively. To this we added 10% for several practical reasons: First, different types of treatment machines were used over the three decades that our investigation spanned (x-ray tubes, cobalt machines and linear accelerators). Second, various energies were used, alone or in combination (kilovolts, megavolts, mega-electron-volts). Third, the doses administered were reported in different ways (reference dose, tumour dose, mid-dose). Fourth, the unit of ionizing radiation has shifted from roentgen (the prevailing unit prior to the introduction of megavoltage machines) to rad to gray, and formulas for the biological effects have shifted from Nominal Standard Dose (N.S.D.) to Cumulative Radiation Effect (C.R.E.) to the Linear-Quadratic model with α and β.
Fifth, the depth of the plexus brachialis from the skin varies over the 10 cm long course of the bundle from the spine to its redistribution into peripheral nerves.
Sixth, the distance of the plexus from the skin varies with the position of the patient under the machine. Finally, overlapping fields may cause considerable over-dosage (an illustration is given below). With all these uncertainties, we preferred to set the final dose at 48.0 Gy EQD2Gy ±10%. Thus, patients receiving total doses lower than 53.0 Gy in biologically equivalent late effects were not considered for indemnification, regardless of the size of the fractions. The limit of 53.0 Gy EQD2Gy automatically excluded all patients who were treated by conventional x-rays (kilovolts), since the skin reaction of the patients usually made doses exceeding 5.000 roentgen (≈ 45 Gy) impossible.
The region of interest in the radiotherapy of breast cancer is large and may encompass the breast, the chest wall, the axilla, and the supraclavicular fossa. Nearby organs are the lungs, ribs, shoulder joint, heart, and – not least – the plexus brachialis.
During this investigation, we focused on the radiation dose to the plexus brachialis, disregarding the dose received by the skin, the breast or the skeleton.
Clinical aspects
All Swedish departments of radiotherapy were asked if they had used hypofractionation later than 1960. Some departments had difficulties in answering the question, since many of the doctors who had been active during the 1960s had either retired or died. Also, some of the departments which offered radio-therapy in the 1960s no longer existed. We were able to locate nine institutions in Sweden which had utilized hypofractionation according to our definition.
The medical records for each patient with cancer of the breast, treated with radiotherapy, were then reviewed by both of us, noting the absorbed doses and fractions, and the reported injuries. For each patient, the treatment plan was reviewed, and in the cases where no plan existed, it was reconstructed. All doses, no matter how they were presented, were recalculated and converted to Equalized Total Dose with 2 Gy/fraction (EQD2Gy). We focused our interest on the brachial plexus and disregarded telangiectasia, ulceration of the skin, or hyper-pigmentation.
Each identified patient was also asked to fill in a questionnaire about her injuries.
The following nine injuries were recorded:
immobilization of the shoulder,
oedema of hand/arm,
pains in the arm,
paralysis of the arm,
fractures in the arm/thoracic skeleton,
heart disease,
respiratory distress,
hoarseness (caused by injuries to the recurrent nerve),
Horner′s syndrome.
The maximum number of points a patient could report was thus 45. Since severe pain in the arm of the treated side almost invariably was accompanied by complete paralysis of the hand and/or arm, a score exceeding 10 points was designed as “severe damage”.
Each patient was also asked to state the latency period between therapy and onset of symptoms.
The subjective grading of the injuries was checked against the information in the medical records. Usually, there was a close correlation between the subjective grading by the patient and the information in the medical record. In cases where there was a large discrepancy between the information given by the patient and that noted in her medical records, the patient was requested to see a medical doctor in order to obtain adjustment.
Definition of brachial plexus neuropathy (BPN)
Injuries of the brachial plexus can be caused by tumours, by surgery, by radiotherapy, and by trauma. In many of the “early” publications on radiation-induced brachial plexopathy (BPN), the information about the accuracy of the diagnosis is frustratingly scanty, or vice versa, if the diagnosis is definitive, the relevant information about the irradiation parameters is often insufficient. There is little value in the statement: “the patient was given radio-therapy” if the basic factors of the treatment are not presented.
It would be as useless information as the statement that:
“the patient underwent surgery”.
Among our patients, the shortest observation period from the onset of symptoms of BPN to the start of this investigation was 15 years. Seven patients in Sweden were treated after 1983; all the others (96%) earlier, rendering the great majority a follow-up period of 23 years without signs of metastases. Even if cancers of the breast (in plural, since cancer of the breast is not one single disease) can be very slow-growing neoplasms, with late recurrences after 15–20 years after removal of the primary Citation[13] we are convinced that the patients in our investigation really have plexus injuries inflicted by radiotherapy.
We accepted the diagnosis of radiation-induced BPN if there were no metastases diagnosed during the 5 years following the onset of symptoms, and also, no intercurrent disease or trauma were reported.
Review of the literature
The Swedish Insurance Company for Patient Injuries asked us to state in what year hypofractionation ought to have been stopped. The reason for this date was to provide a year from which the Company could calculate the period of indemnification. We reviewed the literature. The first eight publications reporting on BPN supposed to be caused by hypofractionation radiotherapy are listed in chronological order in .
Table I. Publications reporting on brachial plexus neuropathy (BPN).
The cumulative number of injured women is exceeding 100 by the year 1968. The international experts who gave their definitions of hypofractionation (see above) were also asked by us to answer a second question: “In what year should the medical profession have realized the hazards of hypofractionation and stopped the treatment?” To facilitate for the experts, all the articles found in of this publication were included in the letter. We received six answers to the second question (but 10 to the first). One expert stated: “They should never have begun”, one said: “after 2–3 papers published in peer review journals”, one gave: “1983”, and three of the experts all gave the year “1968”. We, and the Insurance Company, accepted the year 1968 as the limit for indemnification.
Results
Radiation parameters
Post-operative irradiation was delivered to the axillary region in a variety of fashions. The three most common were:
One single anterior (A) field (17 patients),
One anterior (A) and one posterior field (P) opposing each other (35 patients),
One anterior (A) and one angled (V) right into the axilla (see ) (123 patients).
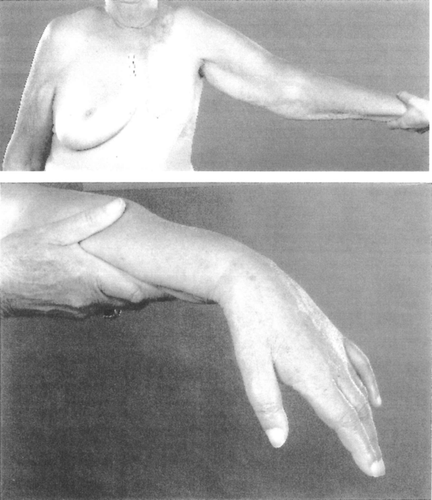
Figure 1. Drop-hand due to radiation-induced brachial plexopathy (BPN). The hand and the arm of the patient were almost completely paralysed, and the muscles atrophied. The patient had also lost all sensory functions in the extremity. This patient had very little lymph oedema.
The size of the individual fractions varied from 2.5 Gy 5 days per week to 6.0 Gy 2 days per week. Total absorbed dose varied from a biological equivalence of 45 to 146 Gy EQD2Gy (6 Gy×13). The highest value for the late effect we have discovered in Sweden is the biological equivalence in late effects of 146 Gy EQD2Gy.
Since the present investigation focuses on injuries to the brachial plexus (BPN), we reconstructed the isodose plans in a few patients for the axillary nodal regions, and also for some of the patients treated with technique 3 above. Since the length of the plexus exposed to irradiation is higher with technique 3 than with the others, more severe injuries could be expected with this technique. The assumption turned out to be correct: Reported injuries related to the treatment technique are clearly indicating that most injuries were caused by the angled field (V): the frequency of injured patients was 71% for those receiving the angled field vs. 29% for those not treated by the angled field.
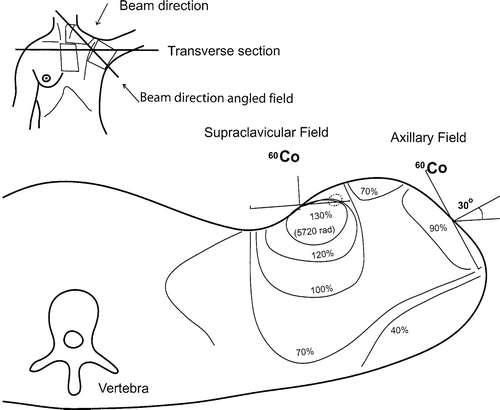
The reconstruction plans indicated that due to cross-fire in the plexus, an overdose of 30% in the axilla, compared to the prescribed dose, was not uncommon. A reconstruction plan is shown in . Individual dose-plans prior to treatment were performed in only 77 of the 175 patients.
Figure 2. A typical dose distribution chart (isodoses) through the centre of the axilla and supraclavicular fields. The patient is viewed from below; the picture displays the left axilla (this is also the way a routine CAT-scan is displayed). Note the 30% overdose compared to the prescribed dose. The icon in the upper left corner shows the patient from the front, with the angled irradiation field indicated. The left breast is missing.
Patient characteristics
We were able to identify 175 patients who fulfilled our four criteria (cancer of the breast + hypofractionated radiotherapy + injury + alive when the investigation started in 2004). Their basic epidemiological data are presented in .
Table II. Basic epidemiologic data of study population.
The most invalidating injury is the damage to the plexus brachialis (BPN). Since we had no possibility to examine any of the patients, the clinical picture – and the latency of injuries – of BPN is based entirely on patient interviews. The results are presented in .
Table III. Frequency of self-reported injuries among 175 women.
The most frequent complaint was a frozen shoulder, almost invariably resulting in a premature and most often involuntary retirement from work. As common as the frozen shoulder is paralysis of the arm on the treated side (see ).
Cardiac morbidity was more frequent in patients receiving radiotherapy to the left side (47%) than to the right (33%). The difference (=14%) is likely to depend on radiation induced damages to the heart. Parestesias (tingling, numbness, etc) were frequently the first symptom, usually in the first two digits of the affected side. Hyperestesia/hypoalgesia followed, indicating that sensory fibres were more susceptible to irradiation than motor fibres. Muscular weakness developed insidiously. Muscular atrophy usually started in the small muscles of the hand and progressed to engage the whole extremity, but very seldom the shoulder girdle. This indicates that the upper plexus (C1–C5) was unharmed. A “drop hand” (see Picture 1) occurred almost invariably. Oedema was frequent, and at times considerable.
Pain could occur very early in the process. Most often it was described as a steady severe ache, in which an intense burning sensation intervened. An increase of the abdominal pressure (i.e. coughing, sneezing or straining) augmented the pain. In many patients, the pain became more intense during – and often preceeded – periods of low atmospheric pressure.
When the BPN was fully developed, the whole extremity had lost most of its sensory and motor functions. Only rarely was the injury confined to the upper or the lower part of the arm.
Excruciating pain and marked oedema (elephantiasis) are prominent parts of the syndrome. The pain is neurogenic (not nociceptive), for which no effective pharmacological analgesics exist.
Once the injuries to the plexus give rise to clinical symptoms, the development is often one of relentless progression.
After many years of muscular atrophy and immobility of the arm, combined with a general oedema of the extremity, the weight of the arm caused a distortion in the humero-scapular joint. In such cases, much of the function of the unaffected arm was occupied by handling the injured arm, rendering the patient almost totally handicapped. Exarticulation occasionally became necessary, much to the practical comfort of the patient (who was relieved of a useless limb). But this drastic measure did not diminish her pain. Healing of the surgical wound was always a very slow process, due to the irradiation.
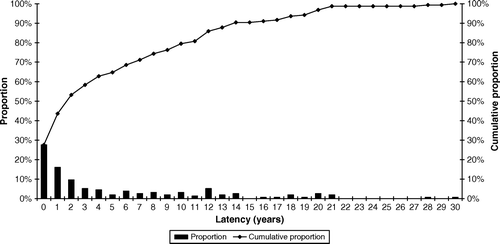
In , the time from completion of radiotherapy to onset of symptoms is shown.
Figure 3. Time from therapy to onset of symptoms Time from end of therapy to onset of symptoms (latency). The diagram points to the fallacy of the use of truncated observation periods, i.e. 5 years. Injuries may appear many years later. Patients in this study with late appearing injuries were often disbelieved and discarded by the medical profession, stating that side effects could not appear after so many years. The diagram can give the visual impression that 100 % of the women treated by hypofractionation are injured. It is the other way round: our study population is selected on the basis of known injuries.
Discussion
Our patient material is in no way representative for women with cancer of the breast in general. It is a highly selected group of patients: Selected on the basis of:
Severe radiation-induced injuries,
hypofractionated radiotherapy, and
still alive at the start of this investigation.
In we listed the eight first publications reporting on BPN. The latest year in that table was 1970.
Since 1970, several reports of radiation-induced injuries to plexus brachialis have appeared. A dissertation by S. Johansson in Umeå, Sweden Citation[23] summarizes in the year 2000 the situation in a very eloquent way. Some of the patients in Johansson's dissertation were originally presented by Westling et al. Citation[19] in 1968. During the 32 years of additional follow-up, almost all surviving patients (92%) had developed severe BPN. The data-base Pub Med today provides over 300 articles dealing with the dangers of hypofractionation Citation[24], Citation[25]. Although the effects of larger doses per fraction can these days be compensated for by reducing total dose very carefully, this was not fully understood in the early 1960s (see Appendix).
One explanation to the differences in the frequency of BPN in various publications may be differences in the length of the follow-up period: many sequels appeared several years or even decades after the radio-therapy. Short follow-up periods will therefore miss some of the side-effects. The tragic results from hypofractionation clearly indicate that peripheral nerves are not resistant to irradiation, at least not with large fractions, as was believed in the 1960s for the reason only explained later by the α/β differences. The belief that peripheral nerves were radioresistant is based on too short follow-up times before clinical trials became formalized.
The fact that not all of the women receiving hypofractionated, high-dose (>60.0 Gy EQD2Gy) radiotherapy developed BPN points to an inter-individual sensitivity to radiation.
We can not give an exact figure for the frequency of injuries to the plexus brachialis in our patient cohort, since it has been impossible to find out the total number of women in Sweden who were treated during the relevant years. (Moreover, we were asked by the Swedish Insurance Company for Patient Injuries to identify only injured patients.)
A guesstimate (guess + estimate) would be 1/3 of all breast cancer patients treated by radiotherapy.
Patients receiving radiotherapy to the left side more commonly developed heart morbidity: 47% treated on the left side compared to 33% in the right. Both figures are high, probably reflecting the high age of this group of patients, at the time when this investigation started (2004).
Apart from the severe damage displayed in this investigation, the long latency period between therapy, and the continuous progression of symptoms and side-effects is perhaps the most striking observation, confirming the observations by several others Citation23–25. At the same time, the long latency period poses a problem to insurance companies who usually have periods of limitations for indemnification set at 5 or 10 years. How handle an injury which becomes evident 15 or 20 years later?
For many of the women in our report, hypofractionated radiotherapy turned their lives into a disaster. They have been physically severely handicapped, some have had their careers ruined, their social relations diminished, some have had their marriages destroyed, and their economy devastated. Most of them have developed excruciating and drug-resistant pain.
Several of these women have been referred to psychiatrists, and considered grumbling whimperers.
Many of the patients with whom we have been in contact during this investigation, state that the size of the monetary indemnification is not so very important; more important is the fact that they are being believed and acknowledged.
“And receive a confession from the medical profession”.
Legal issues
Injuries caused by hypofractionation became a medico-legal issue in some countries. In Norway, where hypofractionated radio-therapy was started as late as 1985 (!), the Government allocated 80 000 000 Norwegian Crowns (700 000 US $) to indemnify the 130 and in 1998 still living women who had been injured by hypofractionation Citation[26]. The results of the Norwegian investigation have neither been published in any medical periodical, nor in any language other than Norwegian.
In Great Britain, several departments of radiotherapy used hypofractionation. Numerous women were injured, and they brought their cases to legal court in 1998. They lost the case since the judge had been convinced that hypofractionation was “common practice”. The verdict of the judge has been published Citation[27], and commented on in a medical publication Citation[28]. It is good that at least some compensation is now being attempted in Sweden, however late and incomparable with suffering.
Acknowledgements
To the internationally well-known experts in radiotherapy who took the time to answer our questions about radiotherapy, and frequently spent time on discussions with us. To Torbjörn Karlberg, Chief Librarian at the Radiumhemmet, The Karolinska University Hospital in Stockholm, for never-failing efforts to find our (sometimes difficult-to-find) references. Declaration of interest: During this investigation, both authors were contracted by the Swedish Insurance Company for Patient Injuries.
Notes
EQD (Equalized Total Dose) is Estro nomenclatura. The corresponding American term is NTD (Normal Total Dose)
References
- Fletcher GH. Regaud Lecture: Perspectives on the history of radiotherapy. Radiother Oncol 1988; 12: 253–71
- Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol 1989; 62: 679–94
- Friberg S, Rudén B-I. Rise and fall of hypofractionation in clinical radiotherapy in the 20th century. Nowotwory 2007; 57: 139–48
- Kim JH, Chu FCH, Hilaris B. The influence of dose fractionation on acute and late reactions in patients with postoperative radiotherapy for cancer of the breast. Cancer 1975; 35: 1583–6
- Thames HD, Withers HR, Peters LJ, Fletcher GH. Changes in early and late radiation responses with altered dose fractionation: Implications for dose-survival relationships. Int J Radiol Oncol Biol Phys 1982; 8: 219–26
- Overgaard M, Bentzén SM, Christensen JJ, Madsen EH. The value of the NSD formula in equation of acute and late complications in normal tissue following 2 and 5 fractions per week in breast cancer patients treated with postmastectomy irradiation. Radiother Oncol 1987; 9: 1–12
- Thames HD, Bentzen SM, Turesson I, Svergaard M, Vanden Bogagt. Time-dose factors in radiotherapy: A review of human data. Radiother Oncol 1990; 19: 219–35
- Wallgren A. Late effects of radiotherapy in the treatment of breast cancer. Acta Oncol 1992; 31: 237–42
- Cox JD. Large-dose fractionation. Cancer 1985; 55: 2105–11
- Fletcher GH. Hypofractionation: Lessons from complications. Radiother Oncol 1991; 20: 10–5
- Fowler, JF. Practical time-dose evaluations, or how to stop worrying and learn to love linear quadratics. In: Technical basis of radiotherapy. SH Levitt, Purdy, JA, Perez, CA, Vijayakumar, S, Springer; 2006. p 3–31.
- Moore NR, Dixon AK, Wheeler TK, Freer CE, Hall LD, Sims C. Axillary fibrosis or recurrent tumour, an MRI study in breast cancer. Clin Radiol 1990; 42: 42–6
- Friberg S, Mattson S. On the growth rates of human malignant tumors: Implications for medical decision making. J Surg Oncol 1997; 65: 284–97
- Mumenthaler v M. Armplexusparesen im Anschluss an Röntgenbestrahlung. Schweiz Med Wschr 1964; 94: 1069–75
- Edelman, AH, Holtz, S, Powers, WE. Rapid radiotherapy for inoperable carcinoma of the breast. Am J Roentgenol 1965:585–99.
- Stoll BA, Andrews JT. Radiation-induced peripheral neuropathy. Brit Med J 1966; 1: 834–7
- Fallet, GH, Moody, JF, Roth, G, Boussina, I. Lésion du plexus brachial survenant aprés radiothérapie pour cancer du sein. Rheumatol 1967;199–204.
- Aullas M. Attiente du plexus brachial chez des sujets porteurs de néoplasie irradiés au cobalt ou en radiothérapie à 200 Kv. Ann de Med Phys 1967; 3: 253–60
- Westling P, Svensson H, Hele P. Cervical plexus lesions following post-operative radiation therapy of mammary carcinoma. Acta Radiol Ther Phys Biol 1972; 11: 209–16
- Frischbier H-J, Lohbeck HU. Strahlenschäden nach Elektrontherapie beim Mammarkarzinom. Strahlenther 1970; 139: 684–94
- Notter G, Hallberg O, Vikterlöf KJ. Stralenschäden am Plexus Brachialis bei Patienten mit Mammarcarzinom. Strahlenther 1970; 139: 538–43
- Montague ED. Experience with altered fractionation in radiation therapy of breast cancer. Radiol 1968; 90: 962–6
- Johansson, S. Late side-effects following radiotherapy after mastectomy in breast cancer patients. A long-term follow-up. Thesis. 2000. Umeå University Medical Dissertations. ISBN 91-7191-877-9.
- Galecki, J, Hicer-Grenkowicz, J, Grudzien-Kowalska, M. Radiation-induced brachial plexopathy and hypofractionated regimens in adjuvant irradiation of patients with breast cancer – a review. Acta Oncol 2006;45:280–4.
- Johansson S. Radiation induced brachial plexopathies. Acta Oncol 2006; 45: 253–7
- Erstatningsutvalget for stråleskader (In Norwegian). Sosial- og Helsedepartementet. Oslo, 1999.
- Breast radiation litigation. Judgement handed down on8th, May, 1998, by The Hon Mrs Justice Ebsworth.
- Dische S, Joslin CAF, Miller S, Bell NL, Holmes JC. The breast radiation injury litigation and the clinical oncologist. Clin Oncol 1998; 10: 367–71
- Withers, HR, Thames, HD, Peters, LJ. Differences in the fractionation response of acutely and late-responding tissues. In Progress in Radio-Oncology.Vol. IIKärcher, et al,New York: Raven Press; 1982. p 287–296.
- Barensen GW. Dose fractionation, dose rate and iso-effect relationships for normal tissue responses. Int J Radiat Oncol Biol Phys 1982; 8: 1981–97
Appendix
This appendix has been written in collaboration with Professors Jack F. Fowler, D.Sc, Ph.D, F. Inst. P., Emeritus Professor of Departments of Human Oncology and Medical Physics, University of Wisconsin, Madison, USA, and Seymour H. Levitt, M.D., D.Sc., Professor Emeritus, University of Minnesota, Foreign Adjunct Professor, Karolinska Institute, Stockholm, Sweden.
The LQ formula reads: BED = D(1 + d/[α/β])
BED = Biological Effective Dose
D = total dose (number of n fractions×size d of each fraction)
d = daily dose (usually given 5 or 6 times a week)
α = Intrinsic radiosensitivity. Loge of the number of cells injured non-reparably per gray of dose of ionizing radiation.
β = Repair capacity. Loge of the number of cells injured in a repairable way per gray squared.
The BED formula is therefore also BED = D×Relative Effectiveness (RE), where RE = the term in the bracket above=(1 + d/[α/β]). This ratio α/β is so important because, taken as a single ratio, it is the only biological factor in the LQ formula, until we come to allow for overall time, not described here.
RE is one of the most useful terms in LQ arithmetic, and it is wise to calculate RE as the first step in any LQ calculation. Then BED is simply “RE multiplied by the total physical dose”. It is an important truth that any given treatment schedule, with n fractions of d Gy = D Gy total dose, will deliver a different biologically effective dose (BED) to Late-reacting tissues from the biologically effective dose (BED) that the same physical dose delivers to Acutely-reacting tissues.
Late-reacting tissues receive a BED of D (1 + d/3), but early-responding tissues receive D (1 + d/10) because of the different α/β ratios. We then specify the Late BEDs in Gy3 and the Early BEDs in Gy10, where the subscripts of each BED tells us which tissue is being discussed in that sentence. This subscript is different from the one used to specify 2 Gy fractions for EQD.
It is easy to convert BED to the LQ equivalent dose in 2 Gy fractions, called EQD (Equivalent Total Dose in 2 Gy fractions), because EQD = BED/RE for 2 Gy fractions, using the relevant α/β. So EQD2 Gy=D(1 + d/[α/β])/(1 + 2/[α/β]). For most Late tissues EQD = BED/1.67 and for most Acute tissues including carcinomas EQD = BED/1.2.
By “hypofractionation” is here meant any fractions exceeding 2.0 Gy. A common example is 4.5 Gy×10 = 45.0 Gy, for which the Late RE is (1 + 4.5/3) = 2.5, yielding a Late BED of 112.5 Gy3 corresponding to an EQD of 112.5/1.67 = 67.5 Gy if in 2Gy fractions; but for the Acute reactions or most tumours the RE is (1 + 4.5/10) = 1.45 so the BED is 45×1.45 = 65 Gy10, corresponding to an EQD of 65/1.2 = 54 in 2 Gy fractions. For these large doses per fraction, the higher biological dose in the late-responding tissues can be seen to be 25% above that of the acute reactions in say skin (67.5/54).
This definition does not take into account the number of fractions per week since the size of the individual fractions and the total dose is more important than the number of treatments per week.
It was not until the early 1960s that the number of fractions and fraction size were considered significant, the number of fractions per week becoming insignificant, and not until about 1980 that the different effects of fractions size on late and early reactions were known, the number per week becoming insignificant Citation[12].
A high early effect is desirable in the tumour cell population, whereas a low late effect is desirable in the normal tissues surrounding the tumour. The early and late effects are alike and accompanying each other up to a total dose of 50 Gy for a wide range of fraction sizes. Above a total dose of 50 Gy, however, the size of each contributing fraction aggravates the late effects more than the early effects.
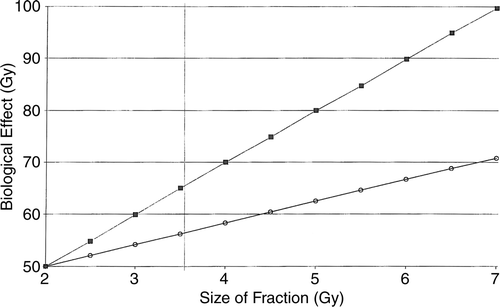
The relationship is illustrated in .
Figure 4. Diagram of acute vs. late effects. Relationship between size of individual fractions (abscissa) vs. corresponding dose in acute and late effects (ordinate) calculated by the linear quadratic formula. The Nominal Total Dose (NTD) is set at 50.0 Gy in this example. At total doses exceeding 53.0 Gy, the late effects increase at a faster rate than the acute effects. Note that the scales are different on the two axes. ▪------------▪: late effect. 0---------0: early effect. Late: α/β = 3 Gy. Early: α/β = 10 Gy. If the size of the fraction is increased from 2.0 to 4.0 Gy, and all other parameters kept constant, the acute effect increases 13%, whereas the late effect increases 40%.
This knowledge was however only being developed in general ways in the late 1960s and 1970s, and the interpretation only became generally recognized in 1980 – 1982. The seminal paper describing this first of all came from Withers, Thames and Peters in 1982 Citation[29]. A paper by Barendsen Citation[30], also seminal and published in 1982, which actually formed the beginning of the application of the LQ formula to radiotherapy, missed this difference in acute/late fraction size effect completely, because it was written in 1980, some months before the papers by Withers et al. Citation[29]. There had been suspicions about injuries caused by hypofractionation before, as discussed in the present paper and Friberg and Rudén Citation[3], but no convincing proof or explanation of the effect.