Abstract
Purpose. Oxypeucedanin has been reported to have various biological activities. We investigated the efficacy of a coumarin compound, oxypeucedanin, from Ostericum koreanum against the human prostate carcinoma cell line DU145. Material and methods. Oxypeucedanin (C16H14O5, mw: 286) was isolated through silica gel chromatography and characterized by NMR. The cells were treated with oxypeucedanin (25, 50, and 100 µM) for 24–72 hours, and cell growth and death were then assayed. The cell cycle progression and apoptotic effects were also assessed by western blotting. Results. Treatment with oxypeucedanin inhibited cell growth and induced cell death in DU145 cells. Furthermore, oxypeucedanin-induced cell growth inhibition was associated with an increase in G2-M arrest in cell cycle progression in DU145 cells in a dose and time-dependent manner. G2-M arrest by oxypeucedanin was associated with decreased levels of cyclin A, cyclin B1, Cdc2, and pCdc2. Oxypeucedanin-induced cell death was associated with significant increases in apoptosis and cleaved caspase-3 and poly-(ADP-ribose) polymerase. Conclusion. These finding suggest a novel anticancer effect for oxypeucedanin, mediated via induction of G2-M cell cycle arrest and apoptosis in human prostate carcinoma DU145 cells.
Ostericum koreanum (Umbelliferae) has traditionally been used in oriental medicine as an analgesic. Isolated imperatorin, isoimperatorin and oxypeucedanin have been reported to have various biological activities including antimutagenic effects Citation[1], Citation[2], causes uterus contraction, increases blood pressure Citation[3], and anticancer effects Citation[4], Citation[5]. It has also been used to treat headache, perspiration, and edema Citation[3].
Prostate cancer (PCA) is the most invasive and frequently diagnosed malignancy in males in the USA and, at present, is the second leading cause of cancer deaths in American males Citation[6], Citation[7]. More than 70% of all prostate cancer cases are diagnosed in men over age 65 in the United States and worldwide Citation[8], Citation[9]. The induction of human prostate cancer has been viewed as a multistage process, involving progression from small, latent carcinomas of low histological grade to large, metastatic carcinomas of higher grade Citation[10]. It is becoming clear that a variety of pathogenic pathways are involved in the genesis of prostate cancer. Among the widely accepted risk factors for prostate cancer are age, race, ethnicity, dietary habits, and androgen secretion and metabolism Citation[11].
We isolated the furanocoumarin compound, oxypeucedanin, from the roots of Ostericum koreanum () and tested its efficacy against a human prostate carcinoma cell line. Using western blotting methods, we investigated its ability to inhibit cell growth and death, to arrest cell cycle progression and to induce apoptosis in the prostate carcinoma cell line DU145.
Material and methods
Plant material, isolation and characterization of oxypeucedanin
The root of Ostericum koreanum (Umbelliferae), which was cultivated in Kangwon Province, Korea, was purchased in March, 2006, and identified by Professor Sookyeon Lee of Sahmyook University. The voucher specimen (SYU-2006-01) was deposited at the Herbarium of Sahmyook University.
Isolation of oxypeucedanin
The air-dried plant material (root, 3 kg) was sliced and extracted with 70% hot MeOH three times for 3 hours each. The extract was filtered and concentrated in vacuo to yield a MeOH extract. The concentrated extract (600 g) was suspended in water and then successively partitioned with n-hexane, EtOAc and n-butanol to afford three fractions and an aqueous residue. The EtOAc extracts were obtained by removal of the solvent. Oxypeucedanin was isolated from the EtOAc fraction by silica gel column chromatography with gradient elution using n-hexane-EtOAc Citation[12]. The compound was characterized by NMR (Bruker AVANCE 400 NMR spectrometer). The purified furanocoumarin compound, oxypeucedanin (C16H14O5) with a molecular weight of 286 (), was dissolved in DMSO as a stock solution that was used directly for cell culture treatments.
Cell line and other reagents
Human prostate carcinoma cell line DU145 was obtained from American Type Culture Collection (Manassas, VA). Cells were cultured in RPMI 1640 with 10% fetal bovine serum (Hyclone, Logan, UT) under standard culture conditions (37°C, 95% humidified air and 5% CO2). RPMI 1640 and other culture materials were purchased from Life Technologies (Gaithersburg, MD). Cleaved caspase-3 and poly (ADP-ribose) polymerase (PARP), cleaved PARP primary antibodies, and peroxidase-conjugated secondary antibody were from Cell Signaling Technology (Beverly, MA). Antibodies to cyclin B, cyclin A, Cdc-25C, Chk1, Chk2, Cdc-2, phospho-Cdc-2, phospho-Cdc-25 were from Santa Cruz Biotechnology (Santa Cruz. CA). Antibody for β-actin was purchased from Sigma (St. Louis, MO). The Annexin V/propidium iodide (PI) staining Vybrant Apoptosis Assay Kit 2 was from Molecular Probes (Eugene, OR). The Enhanced Chemiluminescence (ECL) detection system was from Amersham (Arlington Heights, IL). Other chemicals were obtained at the highest purity grade commercially available.
Cell growth and death assays
DU145 cells were cultured at 1×105 cells/60-mm cell culture plates under standard culture conditions. After 24 h, cells were fed with fresh medium and treated with dimethyl sulfoxide (DMSO) alone (control) or with 25, 50 and 100 µM oxypeucedanin in DMSO. The DMSO concentration was the same for all treatments and did not exceed 0.1% (v/v). After 24, 48 or 72 h of treatments, cells were collected with brief trypsinization, washed with ice-cold PBS, and counted in duplicate using a hemocytometer. Trypan blue dye exclusion was used to determine viable and dead cells.
Apoptotic cell death assay
To quantify the oxypeucedanin-induced apoptotic death of DU145 cells, annexin V and PI staining were performed in conjunction with flow cytometry, as described previously Citation[13], Citation[14]. Briefly, after treatment of cells with oxypeucedanin at each concentration and time point, both floating and attached cells were collected by brief trypsinization, washed twice with PBS, and subjected to annexin V and PI staining using the Apoptosis Assay Kit. The kit contains recombinant annexin V conjugated to fluorophores and the Alexa fluro 488 dye to provide maximum sensitivity. After staining, flow cytometry was performed for the quantification of both early (annexin V stained cells) and late (annexin V/PI stained cells) apoptotic cells.
Cell lysate preparation
DU145 cells were cultured in RPMI 1640 medium and treated with DMSO control or 25, 50, and 100 µM oxypeucedanin for the desired time. Following oxypeucedanin treatments, total cell lysates were prepared in non-denaturing lysis buffer [10 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 1 mM EGTA, 0.3 mM phenylmethylsulfonylfluoride (PMSF), 0.2 mM sodium orthovanadate, 0.5% NP-40 and 5 U/ml aprotinin]. For lysate preparation, the medium was aspirated and cells were washed twice with ice-cold PBS followed by incubation in non-denaturing lysis buffer for 20 min. The cells were then scraped and kept on ice for an additional 30 min, and finally cell lysates were cleared by centrifugation at 4°C for 30 min in a tabletop centrifuge. Protein concentrations in lysates were determined by the Lowry method using a Bio-Rad DC protein assay kit (Bio-Rad Laboratories, Hercules, CA).
Immunoblot analysis
For immunoblot analyses, 70 – 100 µg of protein lysate per sample were denatured in 2×SDS–PAGE sample buffer and subjected to SDS–PAGE on 12 or 16% Tris-glycine gel as needed. The separated proteins were transferred onto nitrocellulose membranes followed by blocking with 5% non-fat milk powder (w/v) in Tris-buffered saline (TBS, 10 mM Tris, 100 mM NaCl, 0.1% Tween 20) for 1 h at room temperature or overnight at 4°C. Membranes were probed for the protein levels of cyclin A, cyclin B1, Cdc-25C, Cdc-2, Chk1, Chk2, phospho-Cdc-2, phospho-Cdc-25, and β-actin using specific primary antibodies and the appropriate peroxidase-conjugated secondary antibodies; immunoreactive bands were visualized by ECL detection. Similarly, the apoptotic molecules, cleaved caspase-3, total PARP, and cleaved PARP, were probed using their specific primary antibodies, appropriate secondary antibodies, and ECL visualization.
Statistical analysis
Autoradiograms of the immunoblots were scanned using Adobe Photoshop, Adobe System (San Jose, CA). Statistical significance of differences between control and treated samples was calculated by Student's t-test (SigmaStat 2.03). P < 0.05 was considered significant. Unless otherwise mentioned, all data shown for cell growth inhibition, cell death, and quantitative apoptosis are representative of two to three independent studies.
Results
NMR characterization of oxypeucedanin
Oxypeucedanin (): pale yellow needle crystal form. 1H-NMR (400MHz, CDCl3): 1.34 (s, C-4′), 1.40 (s, C-5′), 3.25 (q, C-2′), 4.40, 4.70 (dd, C-1″), 6.30 (d, C-3), 6.93 (br, C-8a), 7.07 (d, C-8), 7.62 (d, C-2’), 8.24 (d, C-4).
Effects of oxypeucedanin on growth and death of DU145 cells
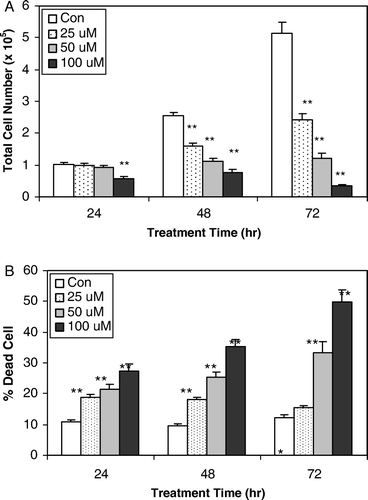
To assess the biological activity of oxypeucedanin in terms of cell growth and death, DU145 cells were treated with 25, 50 and 100 µM oxypeucedanin for 24, 48 and 72 h. Oxypeucedanin (25–100 µM) showed strong dose- and time-dependent cell growth inhibition, accounting for 3.4 to 43.5% (p < 0.001), 8.7 to 76.2% (p < 0.001) and 43.6 to 93.3% (p < 0.001) growth inhibition after 24, 48 and 72 h of treatment, respectively (A). Furthermore, we observed that oxypeucedanin has cytotoxic effects on DU145 cells, such that similar treatment (25–100 µM for 24, 48 and 72 h) with oxypeucedanin caused 15 to 45% (p < 0.001) cell death versus 9.5 to 12.3% in controls (B). Overall, these results suggest that oxypeucedanin has growth inhibitory and cytotoxic effects in human prostate carcinoma DU145 cells.
Figure 2. Growth inhibitory effect and cell death-inducing effect of oxypeucedanin in DU145 cells. 1×105 DU 145 cells were plated in 60-mm dishes for 24 h and then treated with DMSO (0.1%, v/v) or oxypeucedanin (25–100 µM). After 24, 48, and 72 h of these treatments, total cells were counted to assess the growth inhibitory effect (A). Total cells were collected and cell viability was determined by trypan blue dye exclusion method (B). The cell growth and cell death data are shown as the mean±SE of three independent plates for each treatment and each sample was counted in duplicate. *, p < 0.05; **, p < 0.001 versus control.
Oxypeucedanin induces G2-M arrest in the cell cycle progression of DU145 cells
Inhibition of deregulated cell cycle progression in cancer cells is an effective strategy for halting tumor growth Citation[15], Citation[16]. Because we observed a strong inhibitory effect of oxypeucedanin on cell growth, we analyzed its possible inhibitory effect on cell cycle progression at various doses for 24–48 h.
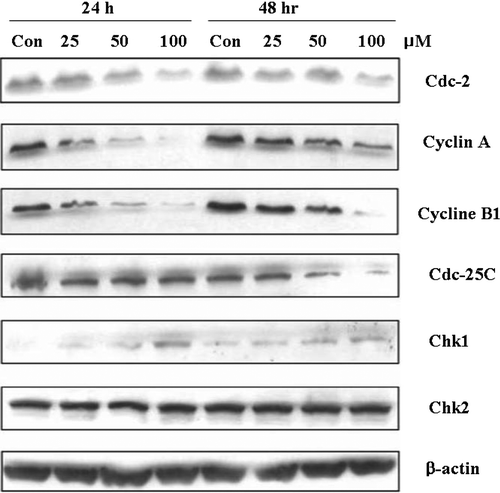
We assessed the effect of oxypeucedanin on G2-M cell cycle regulators including Cdc-25C, Cdc-2, Cyclin A, and Cyclin B1 in DU145 cells (). Oxypeucedanin dose-dependently decreased Cdc-25C protein level at 48 h. Oxypeucedanin also markedly decreased Cyclin A, Cyclin B1, and Cdc-2. However, this agent slightly increased the levels of checkpoint kinase proteins, Chk1 and Chk2 (). Protein loading was checked by reprobing the membranes with antibody against β-actin, the levels of which did not change.
Figure 3. Effects of oxypeucedanin on G2-M cell cycle regulators in DU145 cells. Cells were cultured in RPMI 1640 medium with 10% FBS and treated with DMSO (0.1%, v/v) alone or oxypeucedanin (25–100 µM) for 24 and 48 h. Total cell lysates were then prepared and subjected to SDS-PAGE followed by western blot analysis. Membranes were blotted with anti-Cdc-2, Cyclin A, Cyclin B1, Cdc-25C, Chk1, and Chk2, followed by the appropriate peroxidase-conjugated secondary antibodies, and visualized by ECL detection. Membranes were stripped and reprobed with β-actin antibody as loading control.
The effects of oxypeucedanin on post-translational modifications of the cell division cycle protein phosphatase (Cdc25C) and the mitotic marker protein kinase (Cdk1/Cdc2) were investigated by western blot analysis ().
Oxypeucedanin induces Apoptotic Cell Death of DU145 Cells
During cell growth assays, we observed that oxypeucedanin significantly increased the cell death in DU145 cells, and higher doses and longer treatment times were more effective in regard.
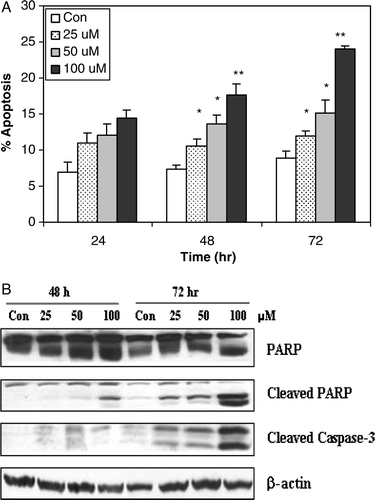
We next examined whether oxypeucedanin-induced cell death was accompanied by induction of apoptosis in DU145 cells (). Cells were treated with 25, 50, and 100 µM oxypeucedanin for 24, 48, and 72 h, and stained with annexin V and PI for observation and quantification of apoptotic cells by fluorescence microscopy. Our data show a dose-dependent increase in the apoptotic cell population following oxypeucedanin treatment. We observed an increase in the apoptotic cell population by 1.5- to 2.0-fold (p < 0.001) and 2.0- and 3.0-fold (p < 0.001) by oxypeucedanin after 48 and 72 h of treatment, respectively. The 24-h treatment slightly increased the apoptotic cell population. However, the lowest dose (25 µM) of oxypeucedanin was not as effective as higher doses (100 µM), which caused more apoptotic cell death (13%) at 72 h than at 24 h of treatment (8%) versus controls, which exhibited 7 to 9% apoptotic cell death (A).
Figure 4. The apoptotic effect of oxypeucedanin on DU145 cells. A. cells were cultured in complete medium and treated with either DMSO vehicle control or 25 to 100 µM oxypeucedanin. After 24, 48, and 72 h, total cells were collected and stained with annexin V/PI followed by flow cytometric analysis. Data are presented as a percentage of annexin V/PI stained cells for each treatment. B. After 48 and 72 h of oxypeucedanin treatment, cell lysates were prepared and SDS-PAGE and western blot analysis were performed for cleaved caspase-3 and total and cleaved PARP using specific antibodies as described in Materials and methods. *, p < 0.05; **, p < 0.001 versus control.
Caspase 3 activation in apoptosis by oxypeucedanin
Activation of the caspase cascade, which leads to PARP cleavage, is regarded as a major pathway in apoptosis induction Citation[17]. Based on the above results showing induction of apoptosis by oxypeucedanin, we measured the levels of cleaved caspase-3 following 48 and 72 h of treatment. Our data showed that oxypeucedanin increased the level of cleaved caspase-3, which was prominent at 100 µM oxypeucedanin (B). Consistent with the cleavage of caspase-3, oxypeucedanin also strongly induced PARP cleavage. We first used PARP antibody, which recognizes both total (116 kDa) and cleaved PARP (89 kDa), and then reprobed with a specific antibody against cleaved PARP that has better sensitivity for the detection of cleaved PARP fragment.
Discussion
In our study, we have identified a novel in vitro anticancer efficacy of oxypeucedanin against human prostate carcinoma DU145 cells. Furthermore, this agent induced apoptosis through a caspase-3-dependent mechanism in DU145 cells.
Coumarin compounds are naturally occurring anti-tumor or anti-inflammatory agents; coumarin also has blood-thinning, anti-fungal and anti-tumor activities Citation[18], Citation[19]. Thus, coumarin should not be taken in conjunction with anticoagulants. Furthermore, coumarin increases the blood flow in the veins and decreases capillary permeability and can be toxic when used at high doses for a long period. Intensive investigation of the hexane soluble fraction of the Ostericum koreanum extract yielded six furanocoumarins, i.e., isoimperatorin, cnidicin, imperatorin, oxypeucedanin, byakangelicol, oxypeucedanin hydrate, all of which significantly inhibited cell proliferation in a dose-dependent manner Citation[19].
In order to evaluate the pharmacological activities of oriental medicines, nine Umbelliferae plants were selected and their restoring activities against dexamethasone-induced disorders, their liver protective activities, their antimicrobial activities, their anti-inflammatory activities, and their antimutagenic activities were tested and compared. Angelica dahurica,A. acutiloba, and Ostericum koreanum showed various activities in these tests at the dose used in this study Citation[20].
In the present study, we evaluated a furanocoumarin, oxypeucedanin, for its ability to inhibit the growth of human prostate carcinoma DU145 cells. We observed that oxypeucedanin inhibited DU145 cell growth in a dose- and time-dependent manner.
To further explore the underlying mechanisms of cell growth inhibition, we conducted cell cycle analyses. We thus elucidated the mechanism by which oxypeucedanin induced G2/M arrest by investigating G2/M cell cycle regulatory proteins. The major regulator of G2-M transition is a complex comprised of catalytic subunit Cdc2 and regulatory subunit cyclin B1 that controls the entry into mitosis Citation[21]. Cdc2 is inactive in its phosphorylated form and is dephosphorylated by Cdc25C phosphatase to form the active complex with cyclin B1 Citation[21], Citation[22]. Consistent with these reports, oxypeucedanin decreased Cdc25C, Cdc2 and cyclin B1 protein levels in DU145 cells ().
We found that oxypeucedanin increased Cdc25C phosphorylation (data not shown), concomitant with the increase of Chk2, which is responsible for Cdc25C phosphorylation. Oxypeucedanin also suppressed the activity of cell division cycle Cdc2 by inhibiting its activator protein phosphatase Cdc25C, since oxypeucedanin decreased Cdc2 activity in a dose-dependent manner. We also investigated the effect of oxypeucedanin on the cyclin partner of Cdc2 kinase (Cyclin B1). We found that oxypeucedanin downregulated cyclin B1 protein in both a time and dose-dependent manner. Overall, the decreases in these proteins could be the underlying molecular events contributing to G2-M arrest by oxypeucedanin in DU145 cells.
Most of the presently available cytotoxic anticancer drugs mediate their effect via apoptosis induction in cancer cells Citation[17], Citation[23], and apoptosis is suggested as one of the major mechanisms for targeted therapy of various cancers including prostate cancer Citation[17], Citation[23]. In the case of advanced prostate cancer, cancer cells become resistant to apoptosis and do not respond to cytotoxic chemotherapeutic agents Citation[24].Therefore, agents that induce apoptotic death of hormone-refractory prostate cancer cells could be useful in controlling this malignancy Citation[25]. Consistent with this approach, our data showing an induction of apoptotic death of advanced prostate cancer cells by oxypeucedanin could be of great significance in identifying the anticancer mechanism (together with cell cycle arrest) of oxypeucedanin for its possible application in prostate cancer control. In this regard, our data show that oxypeucedanin induced apoptosis in DU145 cells, which was also accompanied by PARP cleavage. The cleavage of PARP by activated caspase 3 is considered one of the hallmarks of apoptosis. The increase in the levels of active caspase-3, as well as of cleaved PARP, suggest that caspase activation is an important mechanism in oxypeucedanin-induced apoptosis in prostate cancer cells. However, we did not use all-caspases inhibitor to investigate the involvement of a caspase-independent mechanism in oxypeucedanin-mediated apoptosis of DU145 cells. Further studies are therefore needed to explore the caspase-independent mechanism of apoptosis induction by oxypeucedanin.
In conclusion, our results indicate that oxypeucedanin inhibits human prostate carcinoma DU145 cell growth by G2-M cell cycle arrest, and induces apoptotic cell death. These results also suggest that oxypeucedanin, a coumarin compound from Ostericum koreanum, might be useful as a chemopreventive agent against prostate cancer.
Acknowledgements
This study was financially supported by a grant from Sahmyook University. Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Wall ME, Wani MC, Manikumar G., Hughes TJ, Taylor H, McGivney R, et al. Plant antimutagenic agents, 3. Coumarins. J Nat Prod 1988; 51: 1148–52
- Cai Y, Baer-Dubowska W, Ashwood-Smith M, DiGiovanni J. Inhibitory effects of naturally occurring coumarins on the metabolic activation of benzo[a]pyrene and 7,12-dimethylbenz[a]anthracene in cultured mouse keratinocytes. Carcinogenesis 1997; 18: 215–22
- Chi HJ, Kim HS. Pharmacological study of isoimperatorin and oxypeucedanin. Kor J Pharmac 1970; 14: 21–7
- Oh H, Lee HS, Kim TW, Chai KY, Chung HT, Kwon TO, et al. Furocoumarins from Angelica dahurica with hepatoprotective activity on tacrine-induced cytotoxicity in Hep G2 cells. Planta Med 2002; 68: 463–4
- Kang JY, Lee SY, Yim DS. Effect of isoimperatorin on the proliferation of prostate cancer cell line du145 cells. J Appl Pharmacol 2005; 13: 185–9
- Denmeade SR, Isaacs JT. A history of prostate cancer treatment. Nat Rev Cancer 2002; 2: 389–96
- Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. Cancer statistics, 2005. CA Cancer J Clin 2005; 55: 10–30
- Nelson WG., De Marzo AM, Deweese TL, Lin X, Brooks JD, Putzi MJ, et al. Preneoplastic prostate lesions: An opportunity for prostate cancer prevention. Ann NY Acad Sci 2001; 952: 135–44
- De Marzo AM, Meeker AK, Zha S, Luo J, Nakayama M, Platz EA, et al. Human prostate cancer precursors and pathobiology. Urology 2003; 62: 55–62
- Godley PA, Campbell MK, Gallagher P, Martinson FEA, Mohler JL, Sandler RS. Biomarkers of essential fatty acid consumption and risk of prostatic carcinoma. Cancer Epidemol Biomark Prev 1996; 5: 889–95
- Ross RK, Henderson BE. Do diet and androgens alter prostate cancer risk via a common etiologic pathway?. J Natl Cancer Inst 1994; 86: 252–4
- Baek NI, Ahn Em, Kim HY, Park YD. Furanocoumarins from the root of Angelica dahurica. Arch Pharm Res 2000; 23: 467–70
- Tyagi A, Bhatia N, Condon MS, Bosland MC, Agarwal C, Agarwal R. Antiproliferative and apoptotic effects of silibinin in rat prostate cancer cells. Prostate 2002; 53: 211–7
- Singh RP, Agrawal P, Yim D, Agarwal C, Agarwal R. Acacetin inhibits cell growth and cell cycle progression and induces apoptosis in human prostate cancer cells. Structure-activity relationship with linarin and linarin acetate Carcinogenesis 2005; 26: 845–54
- Grana X, Reddy EP. Cell cycle control in mammalian cells: Role of cyclins, cyclin dependent kinases (CDKs), growth suppressor genes and cyclin-dependent kinase inhibitors (CKIs). Oncogene 1995; 11: 211–9
- Singh RP, Dhanalakshmi S, Agarwal R. Phytochemicals as cell cycle modulators–a less toxic approach in halting human cancers. Cell Cycle 2002; 1: 156–61
- Lowe SW, Lin AW. Apoptosis in cancer. Carcinogenesis 2000; 21: 485–95
- Lee YY, Lee S, Jin JL, Yun-Choi HS. Platelet anti-aggregatory effects of coumarins from the roots of Angelica genuflexa and A. gigas. Arch Pharm Res 2003; 26: 723–6
- Kim YK, Kim YS, Ryu SY. Antiproliferative effect of furanocoumarins from the root of Angelica dahurica on cultured human tumor cell lines. Phytother Res 2007; 21: 288–90
- Kim CM, Heo MY, Kim HP, Sin KS, Pachaly P. Pharmacological activities of water extracts of Umbelliferae plants. Arch Pharm Res 1991; 14: 87–92
- Yuan J, Yan R, Kramer A, Eckerdt F, Roller M, Kaufmann M, et al. Cyclin B1 depletion inhibits proliferation and induces apoptosis in human tumor cells. Oncogene 2004; 23: 5843–52
- Graves PR, Yu L, Schwarz JK, Gales J, Sausville EA, O'Connor PM, et al. The Chk1 protein kinase and the Cdc25C regulatory pathways are targets of the anticancer agent UCN-01. J Biol Chem 2000; 275: 5600–5
- Gurumurthy S, Vasudevan KM, Rangnekar VM. Regulation of apoptosis in prostate cancer. Cancer Metastasis Rev 2001; 20: 225–43
- Pilat MJ, Kamradt JM, Pienta KJ. Hormone resistance in prostate cancer. Cancer Metastasis 1998–1999; 17: 373–81
- Kantoff PW. New agents in the therapy of hormone-refractory patients with prostate cancer. Semin Oncol 1995; 22: 32–4