Abstract
Background. Metastatic cancer from an unknown primary tumour (CUP) is a common and heterogeneous clinical entity. In Sweden like in many other countries, the continuum of care for such patients remains to be established. Material and methods. Data on CUP cases reported to the Swedish Cancer Registry during 1960 through 2007 was used to assess time trends for incidence, survival, and histological tumour type. Results. There was an upward trend for the age-adjusted incidence until the late 1990s. This roughly paralleled the increase for all reported cancers during the same period. The increase of CUP mainly concerned patients aged above 50 years, and tumours classified as adenocarcinomas. The relative survival at 12 months after a diagnosis of CUP was estimated at 20%. However, after 12 months the relative survival levelled of and the 5-year estimate was 10–15% which suggests that a small proportion of CUP cases may be cured. Relative survival was highly dependent on age with substantially better outcome for young patients, particularly those aged below 30 years. We observed no time trend for survival. Discussion. Cases diagnosed as CUP includes patients with metastatic spread from a wide variety of tumours although certain tumour types probably are overrepresented, for example, cancers in sites that are difficult to examine clinically. Incidence trends probably represent the sum of trends for these cancers as well as diagnostic trends. The decreased incidence observed during the last decade might thus be explained in terms of a combination of improved diagnostic methods to detect primary tumours and decreasing incidence for e.g. pancreatic cancer and lung cancer among males. There is a need of evidence-based programs that define the continuum of care for CUP patients. Such a program is currently being developed through collaboration between all health care regions in Sweden.
For most patients who present with a disseminated malignant disease, a routine clinical work-up will usually disclose the underlying primary tumour. However, among 5–10% of all cancer patients, the primary site remains undetected Citation[1]. Variations in this proportion depend probably to some degree on local traditions regarding the extent of routine clinical investigations and the frequency of autopsies, but may possibly also reflect the completeness and validity of cancer registration. The annual age-adjusted incidence in the US has been estimated at 7–12 per 100 000 compared to 6 per 100 000 in the Netherlands Citation[2], Citation[3]. In the US CUP cases represent one of the ten most frequent malignancies and are the fourth most common cause of cancer death.
“Cancer with an unknown primary tumour” (CUP) is thus a common diagnosis. For malignant diseases, prediction of survival and choice of therapy is typically based on the primary tumour. An unknown primary site implies major diagnostic and therapeutic challenges. Establishing the primary site may permit prevention of complications related to the primary tumour, such as bowel obstruction. These circumstances frequently lead to an exhaustive and often fruitless diagnostic search for the primary tumour. The diagnostic strategy for these patients must take into account that most patients are elderly, have a poor prognosis, and that an extensive clinical work-up that may drag on for months may in the end not benefit the patient.
No Swedish health care region has defined the continuum of care for CUP patients so the responsibility for the clinical work-up, treatment and follow-up is often unclear. Many CUP patients often feel ignored by health care as many hospital departments do not consider themselves responsible for CUP patients. In addition, the available literature on CUP provides relatively little guidance on evidence-based care.
This paper provides information on incidence and survival trends for this ill-defined disease entity based on data from the Swedish Cancer Registry during 1960 through 2007. The purpose was to provide a foundation for an evidence-based program that defines the continuum of care for CUP patients. Such a program is currently being developed through collaboration between all health care regions in Sweden.
Material and methods
Since 1958 all patients with a cancer diagnosis must be reported to the Swedish Cancer Registry by the responsible clinician as well as the pathologist/cytologist involved in the diagnostic work up. The coverage of diagnosed cancer cases in the registry has been estimated at 96%. Registration and coding is done according to internationally accepted rules (International Classification of Disease, Injuries and Deaths, ICD). Over the years different versions of the ICD have been used: ICD-7 during 1958 through 1986, ICD-9 during 1987 through 1992, and ICD-O since 1993. However, codes according to the newer classifications are routinely translated back to ICD-7 to enable analyses of time trends. Each registered case is identified by a personal identification number that is unique to all individuals living in Sweden. Multiple primaries in one individual are recorded as separate cases in the incidence statistics Citation[4], Citation[5].
For the purposes of this paper we selected all cases of CUP (ICD-7: 199) reported to the Swedish Cancer Registry during 1960 through 2007.
Age-specific incidence rates (<30 years, 30–40 years, 50–79 years, 80 years and older), and age-standardized total incidence rates during 1960 through 2007 were calculated. Age-standardization was done with the direct method using the 1970 Swedish census population as reference. Age standardized rates were also calculated for histological subtypes (adenocarcinoma, squamous cell carcinoma, undifferentiated carcinoma, and other types), and for each of the six Swedish health care regions (South, South-East, West, North, Uppsala-Örebro, and the Stockholm-Gotland region).
The Cancer Registry files were matched to the Swedish Cause-of-Death Registry 1960 through 2007 by means of computerized record linkage. The linkage was done using the unique ten digit identification number. From the Cause-of-Death Registry we obtained the date of death. A record linkage was also done to RTB (registry over the total Swedish population) to verify that those not registered as dead were actually alive at the end date for follow-up, that is, December 31, 2007.
Survival from all causes of death was calculated from the date of diagnosis using actuarial methods. The cancer-specific survival was obtained by calculating the relative survival which is defined as the ratio between the observed and the expected survival of an age-matched general Swedish population.
Results
During 1960 through 2007 a total of 57 638 cancer cases without a known primary were reported to the Swedish Cancer Registry. There were slightly more female (31 815, 55%) than male cases. The number of new CUP cases reported in 1960 was 690 compared to 1 507 in 2007.
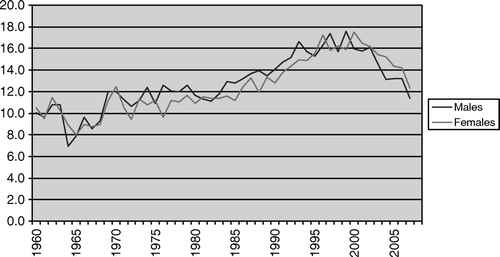
The age-standardized incidence among both males and females increased from c. 10 per 100 000 during the early 1960s to c. 16 per 100 000 around the year 2000. Thereafter it decreased and by 2007 it was c. 12 per 100 000 ().
Figure 1. Age standardized incidence (per 100 000) of cancer cases with an unknown primary tumour in Sweden during 1960–2007.
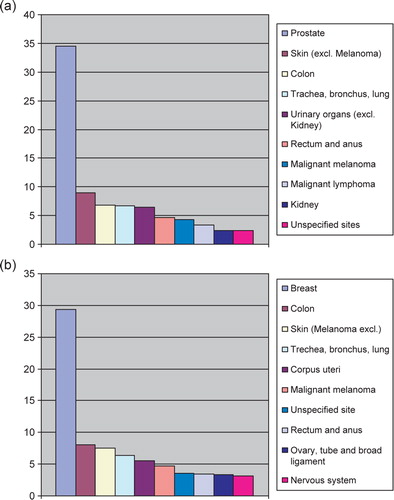
The percentage distribution of the ten most frequent form of cancer among males and females in Sweden 2006 is given in a and b. Cancer cases without a know primary is number seven among males compared to ten among females.
Figure 2. (a) Percentage distribution of the ten most frequent forms of cancer among males in Sweden 2006. (b) Percentage distribution of the ten most frequent form of cancer among females in Sweden 2006.
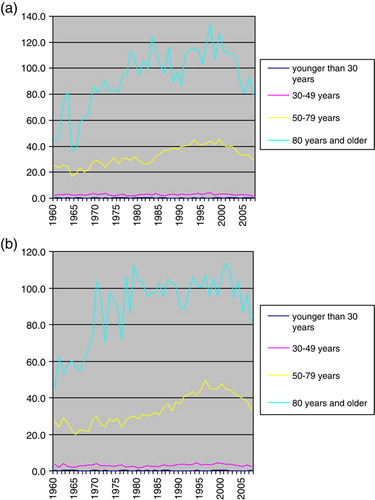
The incidence was consistently higher in older age-groups among both males and females. The increase in incidence over time mainly concerned older age groups, particularly those aged above 50 years. However, for these age groups there was a decrease in incidence since the late 1990s. Among younger age-groups the incidence remained fairly stable (a and b).
Figure 3. (a) Age standardized cancer incidence of CUP among males in Sweden 1960–2007, the rates were standardized for age in each age-category. (b) Age standardized cancer incidence of CUP among females in Sweden 1960–2007, the rates were standardized for age in each age-category.
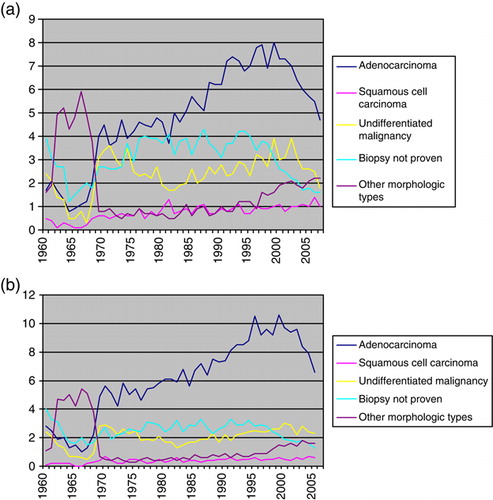
An analysis of incidence trends by histological subtype (a and b) revealed an approximately three to four fold increase of adenocarcinoma among both males and females. The incidence of cases without biopsy proven malignancy remained stable until year 2000. Since then the incidence of such cases has decreased.
Figure 4. (a) Age standardized incidence of CUP among males 1960–2007 by morphologic type. (b) Age standardized incidence of CUP among females 1960–2007 by morphologic type.
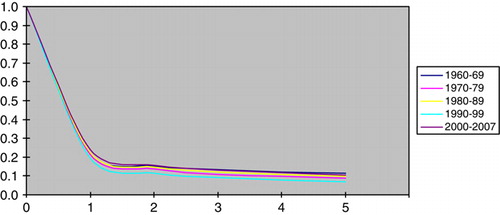
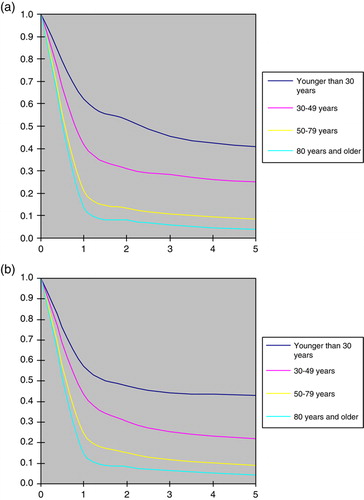
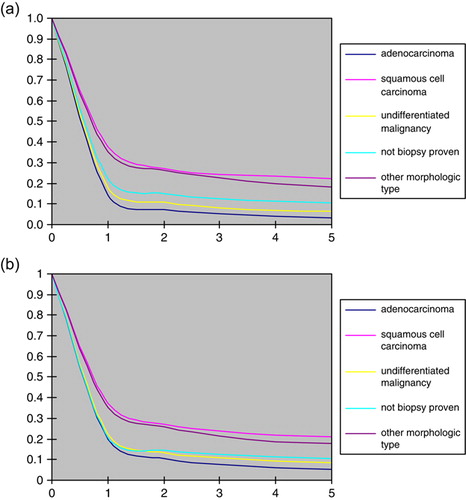
Relative survival was about the same irrespective of the period of diagnosis () whereas it was consistently better at younger ages among both males and females (a and b). Among cases aged below 30 years, for example, the five year survival was estimated at c. 45% compared to c. 5–10% among those aged above 50. The survival difference between the age groups was established during the first year of follow-up, whereas there was relatively little difference in survival thereafter. The relative survival among cases with adenocarcinoma and undifferentiated tumours was markedly poorer than for cases with squamous cell carcinoma (a and b)
Figure 7. (a) Relative survival among males by morphologic type. (b) Relative survival among females by morphologic type.
There were only minor differences in incidence and relative survival between the different geographical regions (data not shown).
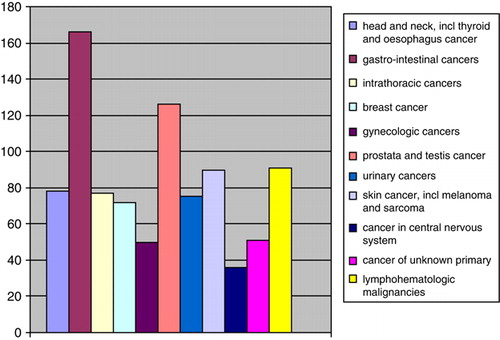
About 2% of the patients were reported to the Cancer Registry because of a new malignancy within 5 years after their initial diagnosis of CUP. Most of these cases concerned malignancies with defined primary sites, . The three most common sites (in descending order) were gastrointestinal cancer, prostate cancer, and lymphohematologic malignancies.
Discussion
Cases diagnosed as CUP probably includes patients with metastatic spread from a wide variety of tumours and, consequently, with diverse etiology and biology. It thus constitutes a separate clinical disease entity mainly from a clinical point of view. Any type of malignancy could theoretically be diagnosed as CUP but it is reasonable to assume that certain tumours are overrepresented, for instance, primary malignancies in internal organs that are difficult to examine clinically. Also, unavailability of specific biomarkers that might direct the clinical work-up could also increase the risk of a malignancy being diagnosed as CUP Citation6–8.
These assumptions were supported by our observation that a gastro-intestinal cancer was the most common primary tumour among those patients for whom a specific diagnosis was reported to the Cancer Registry within five years of their initial diagnosis (). However, tumours among CUP patients may also share certain biological properties, such as acquiring metastatic potential relatively early during tumour progression when the primary tumour is still small and difficult to detect clinically. They also probably include those uncommon cases where the primary tumour has regressed spontaneously, for instance, through immunological mechanisms.
Given the diverse nature of CUP incidence trends for this entity represent the sum of trends in underlying onset rates for many different types of cancer as well as the net effect of diagnostic trends. Such trends may counterbalance each other. For example, an upward incidence trend for lung cancer would probably also increase the incidence of lung cancers being diagnosed as CUP. However, improved methods to detect lung cancer would tend to decrease the risk of a small primary tumour remaining occult and would, therefore, tend to decrease the incidence of CUP.
Also, comparisons of CUP survival rates during different time periods may be biased. For instance, if a specific type of malignancy with a comparatively poor prognosis becomes less prevalent over time among CUP patients (as a result of a true decrease in the underlying onset rate or improved methods to detect occult primary tumours), this would result in a upward survival trend among the remaining CUP patients. Although such a trend may accurately describe the outcome for those classified as having CUP in different time periods, it may not be a valid measure of the secular trend for treatment efficacy.
The main observation reported in this paper was that the age adjusted incidence of CUP increased among both sexes until the late 1990s: in the early 1960s the incidence was about 8–10 per 100 000 compared to 16–17 per 100 000 around the year 2000. The increase mainly concerned those aged above 50 years and tumours classified as adenocarcinomas. In relative terms the increase of CUP cases roughly paralleled the increase observed for all cancer sites during the same period Citation[5].
However, since the late 1990s the incidence trend for CUP cases has shifted downwards. The most recent data from the Swedish Cancer Registry indicated an incidence of about 12 per 100 000. Downward trends for CUP have also been reported from other western countries Citation[9]. In contrast, the age-adjusted incidence for all cancer sites in Sweden continued to increase during the last decade Citation[5].
The decrease in CUP incidence might be explained in terms of improved diagnostic methods, for instance, through a more widespread use of modern imaging techniques, such as NMR, and introduction of immunohistochemical markers in histopathology. Other explanations include downward trends for tumour sites that are overrepresented among CUP cases. For instance, the incidence of pancreatic cancer has decreased since the early 1970s. This decrease has concerned both sexes but has been most prominent among males whose risk in recent years is only about half compared to that during the early 1970s. Lung cancer incidence among males peaked in the late 1970s and has decreased since then. Decreased incidence trends have also been observed for stomach cancer, liver cancer, biliary cancer, and kidney cancer Citation[5].
The increased incidence of CUP mainly concerned tumours classified as adenocarcinoma which also is the dominant histopathology among CUP patients. The fact that some tumour types, such as, gastrointestinal cancers, probably are overrepresented among CUP cases, may help to explain this observation since adenocarcinoma is a common diagnosis among such tumours. Another possibility is that the proportion of adenocarcinomas has increased among tumour types giving rise to CUP cases. Such as shift – the cause of which remains unknown – has been reported among patients with oesophageal and gastric cardia cancer Citation[10] as well as among lung cancer cases Citation[11]. One may speculate that it might be related to shifts in population exposure to etiologic factors. For instance, as a result of the decrease in cigarette smoking among males and the ensuing decrease in lung cancer incidence, an increased proportion of male lung cancer may be due to relevant exposures other than cigarette smoke. Different carcinogenic agents may theoretically result in tumours of different histopathological subtypes.
The survival following a diagnosis of CUP remains relatively short. The relative survival rate at 12 months was estimated at around 20%, and we observed no time trend toward improved results during more recent years. However, it should be pointed out that young patients fared substantially better: those aged below 30 years had a relative survival at 12 months of nearly 70%, and a five year relative survival of more than 40%. Cases with squamous cell cancer and specific histopathologic diagnoses other than adenocarcinoma also fared substantially better than those with undifferentiated malignancies or adenocarcinomas. This observation is in line with reports indicating a worse prognosis for lung cancer patients with adenocarcinomas compared to squamous cell cancers Citation[12], Citation[13].
After the first 12 months the relative survival curve levelled off at 10–15% which is consistent with a small proportion of patients who may have been cured. The diagnosis of CUP among such cases may have concerned regional spread from an occult primary tumour where the primary treatment of the clinically evident disease was able to eradicate also the primary. Another possibility, although more speculative, is that the primary tumour may have undergone spontaneous regression, possibly through immunological mechanisms.
We found that about 2% of the patients were reported with a new cancer diagnosis within five years from the initial diagnosis of CUP. The new diagnosis typically concerned cancer with a defined primary site (). It seems reasonable to assume that most of them constitute patients in whom the primary tumour had been detected so the initial CUP diagnosis should have been removed from the registry. The distribution of cancer sites among these patients probably illustrates which primary sites are common among CUP patients. In this material the three most prevalent diagnoses were gastrointestinal cancer, prostate cancer, and lymphohematologic malignancies.
Patients with CUP represent a significant challenge to both the responsible clinician and the health care sector as a whole. Diagnostic and therapeutic strategies must take into account the fact that many CUP patients are old and have a poor prognosis which speaks against time-consuming, exhaustive investigations which in the end may not benefit the patient Citation[8], Citation[14], Citation[15]. On the other hand, knowledge of the primary site may permit a more specific and effective treatment as well as prevention of local complications from the primary tumour. There is clearly a need of evidence-based programs that define the continuum of care for CUP patients. Such a program is currently being developed through collaboration between all health care regions in Sweden.
Acknowledgements
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Daugaard G. Unknown primary tumours. Cancer Treat Rev 1994; 20: 119–47
- Greco FA, Hainswoth JD. Cancer of unknown primary site. Cancer: Principles and practice of oncology4th ed, TV De Vita, S Hellman, SA Rosenberg. J. B. Lippincott Co, Philadelphia 1997; 2423–2443
- Visser O, Coebergh JWW, Schouten LJ. Incidence of cancer in the Netherlands 1993. The Netherlands Cancer Registry, Utrecht 1993
- Cancerregistret. Cancer incidence in Sweden. 2006. www.Socialstyrelsen.se.
- Cancerregistret. Cancer incidence in Sweden. 2007. www.Socialstyrelsen.se.
- Pavlidis N. Forty years experience of treating cancer of unknown primary. Acta Oncol 2007; 46: 592–601
- Abruzzese JL, Abbruzzese MC, Hess KR, Raber MN, Leugi R, Frost P. Unknown primary carcinoma: Natural history and prognostic factors in 657 consecutive patients. J Clin Oncol 1994; 12: 1272–80
- Randén M, Lewin F, Helde-Frankling M, Johansson H, Lagerros C, Rutqvist LE. Cancer with unknown primary-implementation of a regional referral process and clinical practice guidelines. Clin Oncol 2008; 20: 564
- Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin 2003; 53: 5–26
- Falk J, Carstens H, Lundell L, Albertsson M. Incidence of carcinoma of the oesophagus and gastric cardia. Changes over time and geographical differences. Acta Oncol 2007; 14: 1–5
- Brooks DR, Klint A, Dickman PW, Ståhle E, Lambe M. Temporal trends in non-small lungcancer survival in Sweden. Br J Cancer 2007; 12: 519–22
- Janssen-Heijnen MLG, Scipper RM, Klinkhamer PJJM, Crommelin MA, Mooi WJ, Coebergh JW. Divergent changes in survival for histological typer of non-small-cell lung cancer in southeastern Netherlands since 1975. Br J Cancer 1998; 77: 2053–7
- Kato I, Tominaga S, Ikarl A. Lung cancer prognostic factors from the AICHI Cancer Registry. Jpn J Clin Oncol 1990; 20: 238–45
- Lembersky BC, Thomas LC. Metastases of unknown primary site. Med Clin North Am 1996; 80: 153–71
- Shildt RA, Kennedy PS, Chen TT, et al. Management of patients with metastatic adenocarcinoma of unknown origin: A Southwest Oncology Group study. Cancer Treat Rep 1983; 67: 77–9