Abstract
Purpose. Zoledronic acid is widely accepted as the treatment of choice for a number of cancers which metastasise to bone and is the only bisphosphonate licensed for the treatment of prostate cancer. However, drug related nephrotoxicity, although rare, does pose a significant complication when using zoledronic acid. Prostate cancer patients are generally older than 65 years of age and already exhibit some form of impaired renal function. Thus, for prostate cancer patients who are unable to tolerate zoledronic acid there is a need for an alternative bisphosphonate. One possibility could be ibandronate which is also a potent third generation, nitrogen-containing bisphosphonate and is an attractive choice for some patients due to the fact it is available in both intravenous and oral preparations. Methods. This article reviews the current published literature regarding the use of ibandronate in the treatment of metastatic prostate cancer. Results. Preliminary data emerging from small Phase II studies suggests ibandronate may provide a therapeutic alternative for the treatment of metastatic prostate cancer when zoledronic acid is deemed unsuitable. Conclusion. Further in vivo research with ibandronate in prostate cancer is urgently needed in order to elucidate whether this bisphosphonate may play a role in the treatment and palliative management of metastatic prostate cancer.
Worldwide, it is estimated that greater than 1.5 million cancer patients have bone metastases Citation[1] with breast and prostate cancer accounting for more than 80% of the cases Citation[2]. Since their introduction in the mid-1990s, bisphosphonates have been used for their therapeutic benefits in reducing osteoporosis, fractures, spinal cord compression and other conditions of bone fragility in patients with malignant bone disease (MBD). As a result, bisphosphonates have become part of the standard of care when treating MBD.
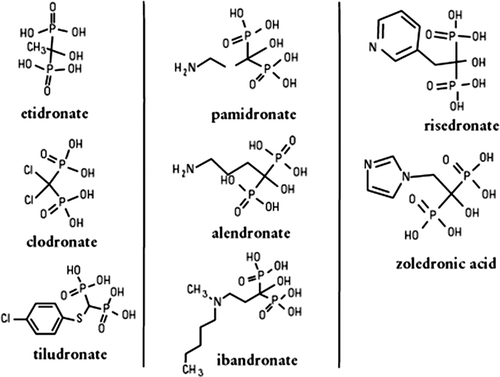
Bisphosphonates are analogues of the naturally occurring pyrophosphate, a substance that inhibits mineral deposition. The phosphoanhydride linkage (P-O-P) is replaced by a non-hydrolysable P-C-P bond. This P-C-P backbone and two covalently bound side chains (R1 and R2), give rise to a variety of structures which differ in their pharmacologic properties and potency () Citation[3]. As a result of their side chains, bisphosphonates can be grouped into two classes depending on their mode of action. The first generation are those which lack a nitrogen containing functional group (clodronate, etidoronate) and are metabolised intracellularly into non-hydrolysable ATP analogues Citation[4]. The more recent generation of bisphosphonates contain either a nitrogen-containing aliphatic side chain (pamidronate, alendronate, ibandronate) or a heterocyclic ring (zoledronic acid) Citation[5]. These nitrogen containing bisphosphonates are more potent than their earlier counterparts by several orders of magnitude (). They act on the mevalonate pathway by inhibiting the enzyme farnesyl diphosphate synthase Citation[6] and the post-translational prenylation of small GTPases of the Ras and Rho family Citation[7].
Figure 2. Differences in potency of different classes of bisphosphonates Citation[9].
![Figure 2. Differences in potency of different classes of bisphosphonates Citation[9].](/cms/asset/486acb16-c330-4b6c-bbef-f1e7c12ff672/ionc_a_387646_f0002_b.gif)
Currently four bisphosphonates are used in the clinical setting for treating MBD. These are clodronate, pamidronate, zoledronic acid and ibandronate Citation[8]. For breast cancer patients, oral clodronate, oral or intravenous (i.v.) ibandronate, i.v. pamidronate and i.v. zoledronic acid are recommended in European guidelines Citation[9]. In prostate cancer only zoledronic acid is currently licensed for use Citation[10]. Zoledronic acid has demonstrated the broadest clinical activity and is regarded as the most potent bisphosphonate. It is widely accepted as the treatment of choice and is the benchmark for the prevention of skeletal complications arising from bone metastases Citation[11]. In prostate cancer patients with bone metastases, zoledronic acid reduces skeletal related events Citation[10], Citation[12], Citation[13], prevent bone loss, by increasing bone mineral density and suppress N-telopeptide (NTX) and bone specific alkaline phophatases, which are biochemical markers of bone resorption Citation[14]. In addition, zoledronic acid can also prevent bone loss in non-metastatic prostate cancer patients receiving androgen deprivation therapy Citation[15]. However, a significant side-effect, although considered rare, associated with the use of zoledronic acid is renal toxicity Citation16–18. Nephrotoxicity is potentially life-threatening Citation[19], Citation[20]. In the USA, Chang et al. Citation[21] highlighted 72 cases in which clinicians reported renal failure associated with zoledronic acid therapy to the Food and Drug Administration; 27 patients required dialysis, and 18 died. Other retrospective studies show renal toxicity associated with zoledronic acid Citation[16], Citation[18], Citation[22], Citation[23]. Prostate cancer patients are generally over 65 years of age and usually have pre-existing renal impairment. Thus, for prostate cancer patients who are unable to tolerate zoledronic acid or it is deemed unsuitable due to pre-existing renal impairment there is a need for an alternative bisphosphonate. One possibility could be ibandronate, another third generation bisphosphonate, which was licensed for use in patients with metastatic breast cancer in the European Union in 2003, and is an attractive choice for some patients due to the fact it is available in both i.v, and oral preparations.
Bisphosphonates efficacy and tolerability have primarily been investigated in patients with bone metastases associated with breast cancer (osteolytic lesions) and ibandronate is no exception. However, bisphosphonates are also efficacious in bone metastases associated with prostate cancer (osteolytic lesions) Citation[13], Citation[14], Citation[24]. In order to understand the usefulness of ibandronate in the clinical setting and the need to investigate its efficacy and tolerability in prostate cancer, this article summarises the current breast cancer clinical trials, in vitro data and then reviews the prostate cancer literature to address the question: Is there a role for ibandronate in the treatment of prostate cancer patients with bony metastases?
Efficacy of ibandronate in in vitro and animal models of breast cancer
Like other bisphosphonates, ibandronate inhibits cell growth of breast cancer cells and induce apoptosis Citation25–28. Ibandronate is more potent at inducing apoptosis in long term cell cultures than zoledronic acid Citation[26], as well as exerting synergistic effects with radiation to inhibit breast cancer cell growth Citation[29]. In vivo evidence from rat models show ibandronate can reduce tumour burden by inhibiting growth of cancer cells Citation[30] and significantly reducing the growth of infiltrating cancer cells at metastatic sites Citation[31].
Efficacy of intravenous and oral ibandronate in breast cancer patients
To date, only one phase III clinical trial has been conducted to assess the efficacy of i.v. ibandronate in reducing the incidence of skeletal complications in breast cancer patients. The trial recruited 466 patients who were randomised to a placebo arm (n = 158), 2 mg (n = 154) or 6 mg (n = 154) ibandronate arm every 3–4 weeks up to 2 years and the primary efficacy parameter was the number of 12 week periods with new bone complications, which was expressed as the skeletal morbidity period rate (SMPR). Patients who received 6 mg ibandronate had a significant reduction in the frequency of 12 week periods with SMPR compared with the placebo group. The mean number of new bone events per patient was also significantly lower in the 6 mg ibandronate group versus placebo (2.65 events versus 3.64, p = 0.032) and the time to a first new bone event was also significantly longer in the 6 mg ibandronate arm than for patients receiving the placebo (50.6 weeks v 33.1 weeks, p = 0.018). In addition, patients in the 6mg ibandronate arm showed a significantly improved bone pain score over time compared with the placebo arm Citation[32]. In a pooled analysis of two phase III clinical trials of oral ibandronate, 287 patients were given 50 mg ibandronate/daily while 277 patients received a placebo Citation[33]. Again the primary efficacy parameter was SMPR and for all new bone events SMPR was significantly reduced in the ibandronate group compared to placebo (p = 0.004). Analyses of new bone events showed the mean number of events and the mean number of measurement periods with events per patient were significantly reduced in the ibandronate group when compared with placebo (p = 0.008 and 0.015 respectively). Multivariate Poisson's regression analysis revealed a 38% risk reduction for a skeletal event that was significantly lower than the placebo group (p < 0.0001).
These trials have demonstrated the efficacy of both i.v. and oral ibandronate in reducing skeletal related events in MBD associated with breast cancer. However, does this efficacy and safety translate into the prostate cancer setting?
Efficacy of ibandronate in in vitro and animal models of prostate cancer
There is very limited in vitro data regarding the efficacy of ibandronate. However, three papers of particular interest show ibandronate inhibits growth and induces apoptosis of the prostate cancer cell line PC-3 Citation[34] and prevents prostate cancer cells adhering to bone Citation[35]. In addition, ibandronate has been shown to accumulate in the prostate of rats following subcutaneous administration causing a decrease in the revascularisation of the prostate gland, when rats were stimulated with testosterone, resulting in a reduction in prostate re-growth Citation[36].
Efficacy of intravenous and oral ibandronate in prostate cancer
Two open, non-randomised trials have assessed the clinical efficacy of ibandronate in the management of symptomatic skeletal metastases due to prostate cancer and other urological cancers. In the first study, 25 patients with painful osseous metastases due to hormone refractory prostate cancer were treated with 6 mg ibandronate every 4 weeks. The primary endpoint was pain reduction using a 10-point visual analogue scale. Twenty-three patients (92%) achieved significant reduction in their pain score (6.5–2.0, p < 0.001), 9 (39%) of which were completely pain free Citation[37] (). The second study was conducted to evaluate the tolerability of high dose ibandronate in metastatic urological cancers. Fifty-three patients with either prostate, renal or bladder cancer and with painful osseous metastases were given ibandronate (6 mg) infused over one hour each day for three consecutive days and this regimen was continued at 4 week intervals. Forty-four patients (83%) reported significant improvement in bone pain starting on day 2. Mean bone pain at the start of the study was 6.8 using a VAS score and on day 3 mean bone pain was scored as 2.5 (p < 0.001) Citation[38] and remained below baseline throughout the 20 week study ().
Figure 3. Reduction in bone pain of prostate cancer patients treated with 6 mg ibandronate every 4 weeks Citation[37].
![Figure 3. Reduction in bone pain of prostate cancer patients treated with 6 mg ibandronate every 4 weeks Citation[37].](/cms/asset/d8b5739b-564f-467b-b137-c359b4ecda0c/ionc_a_387646_f0003_b.gif)
Figure 4. Reduction in bone pain in patients with metastatic urological cancers receiving 6 mg ibandronate over 1 hr each day for 3 days. VAS score remained below baseline throughout the 20 week study Citation[38].
![Figure 4. Reduction in bone pain in patients with metastatic urological cancers receiving 6 mg ibandronate over 1 hr each day for 3 days. VAS score remained below baseline throughout the 20 week study Citation[38].](/cms/asset/eefb24e2-de1c-4a30-b53a-a348d8275505/ionc_a_387646_f0004_b.gif)
At present, there is only one double-blind randomised study that has looked at oral ibandronate in prostate cancer patients Citation[39]. Sixteen prostate cancer patients, along with breast cancer (77), myeloma (3) and 14 other patients, were given 5, 10, 20 and 50 mg ibandronate or placebo. The primary endpoint was calcium excretion (UCCR) as well as bone resorption assessed by measurement of the collagen breakdown products pyridinoline (Pyr) and deoxypyridinoline (Dpd) and the N-terminal (NTX) and C-terminal (crosslaps) portions of the collagen cross linking molecules. A statistically significant dose dependent reduction in UCCR (p < 0.001) and biochemical markers of bone resorption (p < 0.0023) was observed as well as in relation to base line values (UCCR = p < 0.001, Dpd = p < 0.0001). Although patient numbers were low, similar results were seen across all tumour types.
Efficacy of i.v. versus oral ibandronate
The very nature of bisphosphonates means they rapidly bind to bone or are excreted, unchanged, in the urine. Thus, the half-life of bisphosphonates in the circulation is between 0.5 and 2 hours Citation[40]. Pharmacokinetic studies show that oral bisphosphonates are poorly and variably absorbed from the gastrointestinal tract (1–10%) Citation[40]. Oral ibandronate has a linear dose-dependent increase in plasma concentration that is non-saturable Citation[41], while peak plasma concentrations of i.v. ibandronate (6 mg) is 1 µM which rapidly declines to 10% of the maximal concentration between 3–8 hours after administration Citation[42]. Mystakidou et al. (2008) are the only authors to carry out a comparison of oral versus i.v. ibandronate in a variety of tumours, one of which was prostate cancer. Patients received either 6 mg i.v ibandronate infused over 15 min every 28 days or 50 mg/day oral ibandronate. After 6 months the size and number of bone metastases were measured by bone scintigraphy and radiography. I.V. and oral ibandronate produced similar responses across all tumour types with 84.6 and 88.5% of patients showing a complete (resolution and recalcification of all osteolytic metastases) or partial (resolution of some but not all existing osteoblastic metastases, or a decrease in the size of measurable osteoblastic metastases or at least partial recalcification of ≥1 osteolytic metastases and no new bone metastases) response. The Median percentage decreases in S-CTX levels were -39% and -35% for i.v. and oral ibandronate respectively. This comparable efficacy may be due to the fact that the bioavailability of a 50 mg (daily) oral dose, and the resulting plasma concentration, matches that achieved by monthly administration of 6 mg i.v. Citation[41].
Safety issues of bisphosphonates
Both types of preparations of bisphosphonates are associated with adverse events. However the severity and nature varies depending on the route of administration. Unfortunately, for some patients, i.v. bisphosphonate therapy proves problematic. Patients run the risk of developing infusion related adverse events such as injection site reaction, flu-like symptoms, joint pain and pyrexia lasting 1–2 days, although usually associated with the first infusion Citation[8]. In addition, peripheral venous access is not always possible. In such instances, central venous catheterization is used and complications following this procedure can increase problems for the patient Citation[43]. All these i.v. infusion related effects further adds to the problems already faced by patients with advanced metastatic cancer Citation[33].
Renal safety
As mentioned above, a significant disadvantage with some i.v. bisphosphonates is the risk of renal impairment. Although this risk is considered rare, it has been reported in 2.6–25% of patients in phase III trials Citation[44]. Renal damage is associated with high doses and short infusion times Citation[8]. Zoledronic acid is administered as a 4mg/15 minute infusion every 3–4 weeks and renal deterioration is the most significant toxicity associated with its use. Serum creatinine monitoring is required before each dose and is contraindicated when renal failure is present. The potential for renal toxicity, therefore, does mean that the usefulness of zoledronic acid is limited in patients with pre-existing renal dysfunction.
In vivo model assessing renal safety of intravenous bisphosphonates
In an in vivo assay to investigate renal damage caused by i.v. administered zoledronic acid and ibandronate, rats were administered the bisphosphonates as a single injection or once every three weeks for 6 months. Intermittent i.v. injections (1 mg/kg) of zoledronic acid induced renal degeneration and single cell necrosis but was not seen in ibandronate treated rats using the same concentration Citation[45]. One explanation for the differences in nephrotoxicity maybe explained due to the dose dependent accumulation and retention of bisphosphonates in the kidney Citation[46] and the half lives of the respective drugs. Zoledronic acid has a half life (in soft tissues) of around 150–200 days Citation[47] while ibandronate has a half life of only 24 days Citation[48].
Renal safety of zoledronic acid in prostate cancer patients
To date, there has only been one placebo-controlled study of men with advanced prostate cancer, receiving 4 mg zoledronic acid via 15 minute infusion. The trial recruited 643 patients who were randomised to a placebo (n = 208), 4 mg (n = 214) or 8/4 mg (n = 221) arm. Thirty-one percent of the placebo arm (n = 65), 38% of the 4 mg (n = 81) and 28% of the 8/4 mg (n = 62) arm completed the 15 month study. Kaplan-Meier estimates of time to first renal function deterioration were determined. Patients who received zoledronic acid had a relative risk ratio of 1.07 (95% CI = 0.46 to 2.47; p = 0.882), compared with patients who received placebo Citation[12], demonstrating that the zoledronic acid treatment arm had a comparable safety profile to the placebo group.
In a separate study of 221 patients with hormone sensitive prostate cancer, patients received zoledronic acid as a 4 mg via a 15-minute infusion every 3 weeks for 1 year. The study revealed mean maximal change in serum creatinine (SCr) level from baseline was 0.3 mg/dl (where notable SCr increases is defined as an increase ≥0.5 mg/dl for patients with normal baseline SCr levels <1.4 mg/dl, an increase ≥1.0 mg/dl for patients with abnormal baseline SCr levels or ≥2 times the baseline value) Citation[49]. However, notably increased SCr levels were reported in 47 patients (21.2%) and for 21 (44.6%) of these patients, treatment was discontinued.
A retrospective, observational analysis assessing the risk of renal impairment in 122 Citation[50] patients with HRPC has also been reported. Renal impairment was assessed using the same parameters as those of Polascik et al. 2005 Citation[49]. A risk factor analysis was conducted using the Andersen-Gill extension to the Cox proportional hazards model. Renal impairment was observed in 23.8% of patients. The risk increased with extended duration of zoledronic acid treatment (36.8%) among patients who received zoledronic acid for 2 years (not statistically significant) and in patients who had prior treatment with pamidronate compared to those who had not (45.5% vs. 19% respectively). Furthermore, 20.8% of the patients who discontinued zoledronic acid did so as a result of renal complications. Although the authors conceded that reducing the dose for patients with mild or moderate renal complications may reduce the incidence of renal impairment, they report results of renal toxicity and drug discontinuation at approved doses of i.v. zoledronic acid.
The placebo-controlled study conducted by Saad et al. Citation[12] shows zoledronic acid to be well tolerated and safe for use in prostate cancer patients, while the studies conducted by Polascik et al. Citation[49] and Oh et al. Citation[50] would appear to contradict this. The latter two studies, however, were not placebo-controlled. There are many reasons for renal deterioration in cancer patients (progressive disease, nephrotoxic chemotherapy agents, post-renal obstruction, lysis syndrome, uric acid and sepsis Citation[51]) and so due to a lack of adequate controls it cannot be concluded that the zoledronic acid treatment caused or contributed to the renal deterioration observed in the latter studies. Nevertheless all three studies were conducted in patients whose serum creatinine levels were defined as normal. Given that some prostate cancer patients have pre-existing renal complications, it would appear that further investigation into the safety of zoledronic acid is required to fully elucidate the extent to which it may play a role in renal dysfunction/deterioration.
Renal safety of ibandronate in prostate cancer patients
Unlike zoledronic acid, data on the renal toxicity of ibandronate is limited to the very few studies investigating its efficacy in HRPC. Although the aforementioned studies were not carried out using placebo-controlled groups, no renal adverse events were reported. What is interesting is that the two phase II trials using loading-dose ibandronate (6 mg given on three consecutive days) showed no renal adverse effects even in patients with pre-existing renal impairment Citation[37], Citation[38] which could make it a suitable alternative option in prostate cancer patients when zoledronic acid is deemed unsuitable.
Other disadvantages of i.v. bisphosphonates
Osteonecrosis of the jaw (ONJ) is clinically diagnosed by exposed bone in the mouth with pain and poor healing of the wound and tends to occur with long-term exposure to bisphophonates Citation[52], although a cause and effect has not been established Citation[53]. Zoledronic acid counts for 43% of all cases published, while intravenous ibandronate accounts for 2% (oral ibandronate accounts for 0.5%) Citation[54]. However, these figures appear to reflect the fact that the majority of patients (myeloma, breast and prostate cancer) have been treated with zoledronic acid and to date, the true incidence is not yet known Citation[20]. Another disadvantage of i.v. administration is the requirement of regular hospital visits, which can place additional burden on both patients and the hospital. In spite of the rapid infusion time of zoledronic acid (15 minutes), the actual administration time is far greater due to set-up requirements, pre-infusion of saline, drug preparation and safety follow-up Citation[43]. A time-and-motion study Citation[55] has been carried out to assess medical resources used when administering either oral ibandronate or i.v. zoledronic acid. With zoledronic acid administration, average clinic time was shown to be 1 hr 25 minutes longer per patient than with ibandronate treatment. Average clinician time was greater with zoledronic acid treatment (47 minutes compared to 32 minutes for ibandronate treatment) as well as average nurse time (19 minutes versus 3 minutes) []. Additionally, it was reported that for some patients requiring infusions, an overnight stay was needed due to transportation issues.
Table I. Mean clinic time assessing the burden of oral ibandronate and i.v. zoledronic acid in Western Europe Citation[55].
Disadvantages of oral bisphosphonates
The main disadvantages with oral bisphosphonates are the poor bioavailability (<5%) and gastrointestinal toxicity such as nausea, vomiting, reflux oesophagisits and diarrhoea. Due to these adverse side effects, patient compliance is an issue. However, data on non-compliance in patients with metastatic bone disease, is lacking and the only available information in the literature comes from clinical trials using oral clodronate Citation[56]. Clodronate, previously the only licensed oral bisphosphonate available, is a low potency first generation drug that requires high doses to achieve clinical efficacy. The large tablet size of clodronate, which makes it difficult for many patients to swallow, compounds the issue of non-compliance. Non-compliance reduces clinical efficacy and increase morbidity, burden of disease and health care costs Citation[56]. Oral ibandronate is a potent third generation bisphosphonate. The dose of ibandronate, therefore, used to achieve an effect is much lower than that of clodronate and only needs to be administered once a day with the tablets being much smaller in size. Thus it would appear that some of the issues surrounding non-compliance with oral clodronate could be disregarded when treating patients with oral ibandronate.
Future studies of ibandronate in prostate cancer
In the UK, along with bisphosphonates, the current treatment for metastatic bone pain is single dose radiotherapy. The RIB trial, which has been developed by the NCRI and funded by Cancer Research UK, is being carried out to determine whether ibandronate, given as a single 6 mg i.v. infusion, can provide comparable relief from bone pain as a single 8 Gy dose of local radiotherapy in patients with primary prostate cancer and localised metastatic bone pain. The trial is still ongoing and it is hoped that accrual will be completed by the end of 2009.
Conclusion
Bisphosphonates still remain the standard of care for treating skeletal complications caused by metastatic bone disease. Zoledronic acid is the only licensed bisphosphonate for the treatment of prostate cancer. A rare but serious side effect associated with zoledronic acid use is nephrotoxicity. Whilst it is not clear how significant or common this problem is, it would appear that for prostate cancer patients, who have pre-existing renal impairment, zoledronic acid maybe deemed unsuitable. In such instances, there is a need for an alternative bisphosphonate. Phase III clinical trials treating MBD from breast cancer patients show ibandronate is highly efficacious and safe. Ibandronate is not currently licensed for use in prostate cancer treatment and data on its use for this disease is severely limited with most studies carried out on small, non-randomised patient populations. However, the preliminary data that is emerging from these few studies appear to suggest that the use of ibandronate in prostate cancer is efficacious and well tolerated with no evidence of renal toxicity, even in patients with pre-existing renal impairment. These preliminary results need to be confirmed in larger randomised and placebo controlled trials to thoroughly investigate ibandronate as a serious treatment option for prostate cancer patients in order to improve the clinical management of their disease.
Acknowledgements
Declaration of interest: Professor John Wagstaff has been an advisor for Roche.
References
- Brown JE, Neville-Webbe H, Coleman RE. The role of bisphosphonates in breast and prostate cancers. Endocr Relat Cancer 2004; 11: 207–24
- Coleman RE. Metastatic bone disease: Clinical features, pathophysiology and treatment strategies. Cancer Treat Rev 2001; 27: 165–76
- Clezardin P. Anti-tumour activity of zoledronic acid. Cancer Treat Rev 2005; 31(Suppl 3)1–8
- Denoyelle C, Hong L, Vannier JP, Soria J, Soria C. New insights into the actions of bisphosphonate zoledronic acid in breast cancer cells by dual RhoA-dependent and -independent effects. Br J Cancer 2003; 88: 1631–40
- Goffinet M, Thoulouzan M, Pradines A, Lajoie-Mazenc I, Weinbaum C, Faye JC, et al. Zoledronic acid treatment impairs protein geranyl-geranylation for biological effects in prostatic cells. BMC Cancer 2006; 6: 60
- Rogers, MJ, Gordon, S, Benford, HL, Coxon, FP, Luckman, SP, Monkkonen, J,, et al Cellular and molecular mechanisms of action of bisphosphonates. Cancer 2000;88(12 Suppl):2961–78.
- Walker K, Olson MF. Targeting Ras and Rho GTPases as opportunities for cancer therapeutics. Curr Opin Genet Dev 2005; 15: 62–8
- Cameron D, Fallon M, Diel I. Ibandronate: Its role in metastatic breast cancer. Oncologist 2006; 11(Suppl 1)27–33
- Aapro M, Abrahamsson PA, Body JJ, Coleman RE, Colomer R, Costa L, et al. Guidance on the use of bisphosphonates in solid tumours: Recommendations of an international expert panel. Ann Oncol 2008; 19: 420–32
- Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst 2004; 96: 879–82
- Smith MR. Zoledronic acid to prevent skeletal complications in cancer: corroborating the evidence. Cancer Treat Rev 2005; 31(Suppl 3)19–25
- Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst 2002; 94: 1458–68
- Saad F, Chen YM, Gleason DM, Chin J. Continuing benefit of zoledronic acid in preventing skeletal complications in patients with bone metastases. Clin Genitourin Cancer 2007; 5: 390–6
- Israeli RS, Rosenberg SJ, Saltzstein DR, Gottesman JE, Goldstein HR, Hull GW, et al. The effect of zoledronic acid on bone mineral density in patients undergoing androgen deprivation therapy. Clin Genitourin Cancer 2007; 5: 271–7
- Smith MR, Eastham J, Gleason DM, Shasha D, Tchekmedyian S, Zinner N. Randomized controlled trial of zoledronic acid to prevent bone loss in men receiving androgen deprivation therapy for nonmetastatic prostate cancer. J Urol 2003; 169: 2008–12
- Johnson, K, Gable, P, Kaime, E, Luiken, G, Castillos, T, Hu, J. Significant deterioration in renal function with the new bisphosphonate, zoledronic acid. Proc Am Soc Clin Oncol 2003;22, Abstract 2968.
- McDermott RS, Kloth DD, Wang H, Hudes GR, Langer CJ. Impact of zoledronic acid on renal function in patients with cancer: Clinical significance and development of a predictive model. J Support Oncol 2006; 4: 524–9
- Markowitz GS, Fine PL, Stack JI, Kunis CL, Radhakrishnan J, Palecki W, et al. Toxic acute tubular necrosis following treatment with zoledronate (Zometa). Kidney Int 2003; 64: 281–9
- Balla, J. The issue of renal safety of zoledronic acid from a nephrologist's point of view. Oncologist 2005;10:306–8, Author reply 11–2.
- Diel IJ, Bergner R, Grotz KA. Adverse effects of bisphosphonates: Current issues. J Support Oncol 2007; 5: 475–82
- Chang, JT, Green, L, Beitz, J. Renal failure with the use of zoledronic acid. N Engl J Med 2003;349:1676–9, Discussion –9.
- Mazj, S, Litchtman, S. Renal dysfunction associated with bisphosphonate use: Retrospective analysis of 293 patients with respect to age and other clinical characteristics. J Clin Oncol 2004;22(22 Suppl 735) Abstract 8039.
- Barot, K, Wu, S, Zhu, X. Risk of renal dysfunction with zoledronic acid in patients with metastatic solid tumours. J Clin Oncol 2008;26(15 Suppl) Abstract 20530.
- Ishizaka K, Machida T, Kobayashi S, Kanbe N, Kitahara S, Yoshida K. Preventive effect of risedronate on bone loss in men receiving androgen-deprivation therapy for prostate cancer. Int J Urol 2007; 14: 1071–5
- Hiraga T, Williams PJ, Mundy GR, Yoneda T. The bisphosphonate ibandronate promotes apoptosis in MDA-MB-231 human breast cancer cells in bone metastases. Cancer Res 2001; 61: 4418–24
- Verdijk R, Franke HR, Wolbers F, Vermes I. Differential effects of bisphosphonates on breast cancer cell lines. Cancer Lett 2007; 246: 308–12
- Journe F, Chaboteaux C, Magne N, Duvillier H, Laurent G, Body JJ. Additive growth inhibitory effects of ibandronate and antiestrogens in estrogen receptor-positive breast cancer cell lines. Breast Cancer Res 2006; 8: R2
- Fromigue O, Lagneaux L, Body JJ. Bisphosphonates induce breast cancer cell death in vitro. J Bone Miner Res 2000; 15: 2211–21
- Journe F, Magne N, Chaboteaux C, Kinnaert E, Bauss F, Body JJ. Sequence- and concentration-dependent effects of acute and long-term exposure to the bisphosphonate ibandronate in combination with single and multiple fractions of ionising radiation doses in human breast cancer cell lines. Clin Exp Metastasis 2006; 23: 135–47
- Neudert M, Fischer C, Krempien B, Bauss F, Seibel MJ. Site-specific human breast cancer (MDA-MB-231) metastases in nude rats: Model characterisation and in vivo effects of ibandronate on tumour growth. Int J Cancer 2003; 107: 468–77
- Neudert M, Fischer C, Krempien B, Seibel MJ, Bauss F. A rapid histological score for the semiquantitative assessment of bone metastases in experimental models of breast cancer. Onkologie 2008; 31: 521–7
- Body JJ, Diel IJ, Lichinitser MR, Kreuser ED, Dornoff W, Gorbunova VA, et al. Intravenous ibandronate reduces the incidence of skeletal complications in patients with breast cancer and bone metastases. Ann Oncol 2003; 14: 1399–405
- Body JJ, Diel IJ, Lichinitzer M, Lazarev A, Pecherstorfer M, Bell R, et al. Oral ibandronate reduces the risk of skeletal complications in breast cancer patients with metastatic bone disease: Results from two randomised, placebo-controlled phase III studies. Br J Cancer 2004; 90: 1133–7
- Dumon, JC, Journe, F, Kheddoumi, N, Lagneaux, L, Body, JJ. Cytostatic and apoptotic effects of bisphosphonates on prostate cancer cells. Eur Urol 2004;45:521–8, Discussion 8–9.
- Boissier S, Magnetto S, Frappart L, Cuzin B, Ebetino FH, Delmas PD, et al. Bisphosphonates inhibit prostate and breast carcinoma cell adhesion to unmineralized and mineralized bone extracellular matrices. Cancer Res 1997; 57: 3890–4
- Fournier P, Boissier S, Filleur S, Guglielmi J, Cabon F, Colombel M, et al. Bisphosphonates inhibit angiogenesis in vitro and testosterone-stimulated vascular regrowth in the ventral prostate in castrated rats. Cancer Res 2002; 62: 6538–44
- Heidenreich A, Elert A, Hofmann R. Ibandronate in the treatment of prostate cancer associated painful osseous metastases. Prostate Cancer Prostatic Dis 2002; 5: 231–5
- Heidenreich A, Ohlmann C, Olbert P, Hegele A. High dose ibandronate is effective and well tolerated in the treatment of pain and hypercalacaemia due to metastatic urological cancer. Eur J Cancer 2003; 1(Suppl 5)S270
- Coleman RE, Purohit OP, Black C, Vinholes JJ, Schlosser K, Huss H, et al. Double-blind, randomised, placebo-controlled, dose-finding study of oral ibandronate in patients with metastatic bone disease. Ann Oncol 1999; 10: 311–6
- Martin T, Grill V. Bisphosphonates – mechanisms of action. Aust Prescrib 2000; 23: 130–2
- Leyland-Jones B. Pharmacokinetic and clinical equivalence of oral and intravenous ibandronate for metastatic bone disease. Eur J Cancer Suppl 2004; 2: 9–12
- Barrett J, Worth E, Bauss F, Epstein S. Ibandronate: A clinical pharmacological and pharmacokinetic update. J Clin Pharmacol 2004; 44: 951–65
- Cameron, D. Patient management issues in metastatic bone disease. Semin Oncol 2004;31(5 Suppl 10):79–82.
- Body JJ. Bisphosphonates for malignancy-related bone disease: Current status, future developments. Support Care Cancer 2006; 14: 408–18
- Pfister T, Atzpodien E, Bauss F. The renal effects of minimally nephrotoxic doses of ibandronate and zoledronate following single and intermittent intravenous administration in rats. Toxicology 2003; 191: 159–67
- Cal JC, Daley-Yates PT. Disposition and nephrotoxicity of 3-amino-1-hydroxypropylidene-1,1-bisphosphonate (APD), in rats and mice. Toxicology 1990; 65: 179–97
- CEDR New and Generic Drug. Approval Zometa (zoledronic acid) injection, Part 2, NDA 21–223, Pharmacol Rev, Pharmacokinet. Summary ( http://www.fda.gov/cder/foi/nda/2001/21-223_Zormeta_pharmr_P2.pdf). 1998.
- Pfister T, Atzpodien E, Bauss F. Preclinical renal safety profile of ibandronate. Bone 2008; 42(Suppl 1)S105–S106
- Polascik TJ, Given RW, Metzger C, Julian SR, Vestal JC, Karlin GS, et al. Open-label trial evaluating the safety and efficacy of zoledronic acid in preventing bone loss in patients with hormone-sensitive prostate cancer and bone metastases. Urology 2005; 66: 1054–9
- Oh WK, Proctor K, Nakabayashi M, Evan C, Tormey LK, Daskivich T, et al. The risk of renal impairment in hormone-refractory prostate cancer patients with bone metastases treated with zoledronic acid. Cancer 2007; 109: 1090–6
- Lameire NH, Flombaum CD, Moreau D, Ronco C. Acute renal failure in cancer patients. Ann Med 2005; 37: 13–25
- Polascik TJ, Mouraviev V. Zoledronic acid in the management of metastatic bone disease. Ther Clin Risk Manag 2008; 4: 261–8
- Carey JJ, Palomo L. Bisphosphonates and osteonecrosis of the jaw: Innocent association or significant risk?. Cleve Clin J Med 2008; 75: 871–9
- Reid IR. Osteonecrosis of the jaw: Who gets it, and why?. Bone 2009; 44: 4–10
- Wardley A, Body JJ, Neary MP. A time-in-motion study of oral ibandronate versus iv zoledronic acid for treatment of metastatic bone disease in breast cancer patients. J Clin Oncol 2005; 23: 16s
- Conte P, Guarneri V. Safety of intravenous and oral bisphosphonates and compliance with dosing regimens. Oncologist 2004; 9(Suppl 4)28–37