Abstract
Background. To analyse the impact of radiation dose escalation and hormone treatment in prostate cancer patients according to risk groups. Material and methods. Totally 494 prostate cancer patients received external beam radiation therapy, with or without androgen deprivation, between January 1990 and December 1999. The patients were divided into three risk groups, where the low risk group (stage T1c, pretreatment prostate-specific antigen (PSA) level ≤10 ng/ml and WHO Grade 1) included 26 patients, the intermediate risk group (either stage T2, PSA 10.1–20 ng/ml or WHO Grade 2) comprised 149 patients whereas the high-risk group (either stage T3, PSA >20 ng/ml or WHO Grade 3) included 319 patients. Results. In the intermediate risk group, the 5-years bNED rate was 92%, 69% and 61% after a radiation dose of 70 Gy, 66 Gy or 64 Gy, respectively (p < 0.001). In the high-risk group, the 5-year bNED rate was 79%, 69% and 34% for the same dose levels (p < 0.001). The 5-years CSS rates were not significantly different between the dose levels in the intermediate risk group while for the high-risk group it was 93%, 92% and 80% for the three dose levels (p < 0.001). Risk group and radiation doses were independent predictors of bNED, CSS and overall survival, for bNED also hormone treatment was independent predictors. Conclusion. Radiation dose is important for the outcome in intermediate and high risk prostate cancer patients. A dose of 70 Gy should be considered the minimal dose for these patients.
Prostate cancer is the most common malignancy in Norway, with more than 4 300 new cases a year Citation[1]. Most patients are now diagnosed at a localized stage as a result of the widespread use of prostate-specific antigen (PSA) testing in Norway since the early nineties.
External beam radiation therapy (RT) has been used as primary therapy for localised prostate cancer for many years in Norway, but was initially regarded as inferior to surgery. However, radical prostatectomy and RT achieved similar rates of biochemical control and cancer specific survival (CSS) in patients with low risk prostate carcinoma Citation[2], Citation[3]. Furthermore, surgery yielded better outcome than watchful waiting (WW) Citation[4] in localised prostate cancer. The current standard definitive therapies for localized cancer prostate are therefore radical prostatectomy or RT (external beam or brachytherapy). Recently the development of three-dimensional conformal and intensity-modulated RT techniques has reduced the therapy-related morbidity and improved the efficacy of RT.
Before we have published the results of our series of prostate cancer where the radiation dose was stepwise increased from 64 via 66 to 70 Gy, we demonstrated a significant improved PSA failure free survival (bNED) and survival in localized prostate cancer with a dose of 70 Gy compared with doses of 64–66 Gy Citation[5]. We here analyse whether the benefit of radiation is found in all defined risk groups.
Material and methods
Patients
Between 1990 and 1999, 502 patients with T1-3NxM0 prostate cancer were treated with radical RT with or without adjuvant/neoadjuvant hormone treatment (HT) at Haukeland University Hospital. In this period the tumour dose was stepwise increased from 64 Gy via 66 Gy to 70 Gy. Eight patients were excluded from the analysis as they for various reasons were given a tumour dose less than 60 Gy, leaving 494 patients eligible for analysis. The patients were staged by physical examination, PSA testing and isotope bone scan. The primary tumour was assigned a T-category based on digital rectal examination Citation[6]. Histology was in the first part of the study based on the World Health Organisation (WHO) histological grading (302 patients) Citation[7], later according to the Gleason scoring system (192 patients) Citation[8]. In the risk group analysis we converted Gleason score into WHO grading: Gleason score 4–6 to well differentiated (Grade 1), Gleason score 7 to moderately differentiated (Grade 2) and Gleason score 8–10 to poorly differentiated (Grade 3) Citation[9]. Patients’ treatment characteristics are summarized in . All patients had a performance status of 0 or 1 and a life expectancy of at least 10 years.
Table I. Patients treatment characteristics.
Radiotherapy and hormonal therapy
All patients underwent external beam RT with individualised treatment planning, using high energy photons to a total tumour dose of 64–70 Gy, in 2 Gy fractions five days a week, over 6–7 weeks. Before 1995 the treatment plan was based on a diagnostic CT with adaptation to the patients contour at simulation, later all treatment plans were based on 3D CT scans from our dedicated CT scanner. All patients were treated with a four-field box technique (opposing anterior-posterior fields and two opposing lateral fields), with wide margins (2 cm) to 50 Gy before a boost with smaller margins were delivered using four fields to a total dose of 64–70 Gy. Field shaping with individually customised blocks was used occasionally in the first part of the study period, and routinely from 1994. In 1996, the use of customised blocks was substituted by the use of a multileaf collimator. In addition, the dose was increased, first to a total dose of 66 Gy and then further increased to 70 Gy in 1997. Details of the 3-D conformal radiotherapy technique has been published elsewhere Citation[10]. In the period 1990–1998 the high risk patients received radiation to the prostate and seminal vesicle to 50 Gy, followed by a 14–20 Gy boost to the prostate only. The low and intermediate risk patients received radiation to the prostate with 2 cm uniform margins to 50 Gy, followed by a 14–20 Gy boost to the prostate with 1 cm uniform margins; elective pelvic lymph node irradiation was not performed in high risk patient before 1999. From 1999, in the low risk patients the clinical target volume (CTV) encompassed the prostate only with 5 mm margin. Two planning target volumes (PTVs) were constructed. The PTV prescribed 50 Gy was constructed by adding 15 mm to the CTV in all directions apart from the rectum where 10 mm margin was added. For the boost PTV that was prescribed 70 Gy a margin of 10 mm was added to the CTV, except towards the rectum where 5 mm was used. The intermediate risk patients, received radiation to the prostate and seminal vesicles to 50 Gy, followed by a 20 Gy boost to the prostate only. The PTV prescribed 50 Gy was constructed by adding first 5 mm to the prostate and seminal vesicles to define the CTV, then an additional 15 mm on to the CTV, except towards the rectum where a 10 mm margin was used. The boost PTV prescribed 70 Gy was defined by adding first a margin of 5 mm to the prostate only to define the CTV, the an additional 10 mm on to the CTV, except towards the rectum where 5 mm was used. High risk patients were treated with modified pelvic fields to 50 Gy, followed by a reduced volume, which encompassed the prostate and seminal vesicle to 20 Gy.
Neoadjuvant and adjuvant HT was used in 402 of the 494 (81%) patients (). In the first part of the study period a luteinizing hormone releasing hormone (LhRh) agonist was used to downstage tumours before RT for an average of 4–6 months. Total androgen blockade (TAB) was used as short-course (≤6 months) treatment in 89% of the cases and as a long-term treatment (between 6 months and 3 years) in 11%. TAB was initiated when the patient was referred for RT, i.e. 8–12 week's prior to start of RT.
Definition of risk factor groups
Three risk groups were defined, with patients in the low risk group having stage T1c disease, a pretreatment PSA level ≤10 ng/ml and a WHO Grade 1 biopsy. In the intermediate risk group, patients had one or more of the following adverse factors: stage T2 disease, PSA >10 ng/ml and ≤20 ng/ml and biopsy WHO Grade 2. Patients in the high-risk group had one or more of the following factors: stage T3 disease, PSA >20 ng/ml and biopsy WHO Grade 3. All 494 patients were eligible for the risk factor selection analysis. The pretreatment PSA of three patients was unknown, all of these patients had a biopsy WHO Grade 3 and were therefore included in the high risk group. The T stage of four patients was unknown, however, all of these had PSA >20 ng/ml and were also included in the high-risk group.
Follow-up
Follow-up was individualised according to the standard health care service, which in general implied routine follow-up at local hospitals. The patients were scheduled to be followed at the department of urology at the local hospital, or as a secondary alternative, with the patient's general practitioner (GP). The frequency of follow-up examinations was left to the responsible urologist, but annual reports were sent to the Department of Oncology, reporting on clinical progression, adverse effects and death. The follow-up included physical examination and serum PSA determinations. For all patients seen at a hospital, the clinical charts and hospital records were reviewed; for patients seen by their GP we had communication with the GP when appropriate. All patients were followed to death or to May 5, 2004.
Progression
PSA level was used as a surrogate endpoint for disease activity. We used the Houston methods which specify that a relapse is scored when PSA is 2 ng/ml greater than the nadir PSA Citation[11]. All patients with a rising PSA above this level were considered as having biochemical failures.
Adverse effects
Because of the previously described follow-up routines, there were reasons to suspect underreporting of adverse effects by controlling clinicians as no formal scoring forms were used. In the present study we therefore focus on the effect on the tumour, while the risk of late effects were prospectively investigated in a separate later cohort of patients Citation[12].
Statistical methods
All statistical analyses were performed with the SPSS statistics package (v 13.0, SPSS Inc., Chicago, USA) and with R (The R Foundation for Statistical Computing, Vienna, Austria). The primary endpoint was CSS, with overall survival (OS) and bNED being secondary endpoints. The time to the relevant events was measured from the start of radiation therapy, analysed by the Kaplan-Meier method and assessed by the log-rank test Citation[13], and multivariate Cox regression models with risk group, radiation dose, hormonal therapy and age as covariates. The Regional Ethics Committee approved the project.
Results
Pre-treatment characteristics
The pretreatment clinical characteristics of the 494 patients are summarised in . The median age was 67 years (range 47 to 85). The mean initial PSA (iPSA) was 15.4 ng/ml (range: 0.5–298 ng/ml). The low risk group comprised 5% of the patients, while 30% of the patients had intermediate risk and 65% had high risk.
Follow-up times
The mean follow-up time for the entire cohort of 494 patients was 6.2 years. For the low risk group the mean follow-up time was 4.5 years (range 0.2–7.5 years), for the intermediate risk group 6.4 years (range 0.7–14.3 years), and for high risk group 6.3 years (range 0.2–14 years).
Biochemical failure
Of the 494 patients, 175 (35%) had a rising PSA at a median interval of 29 months (range 4–150 months) after start of RT.
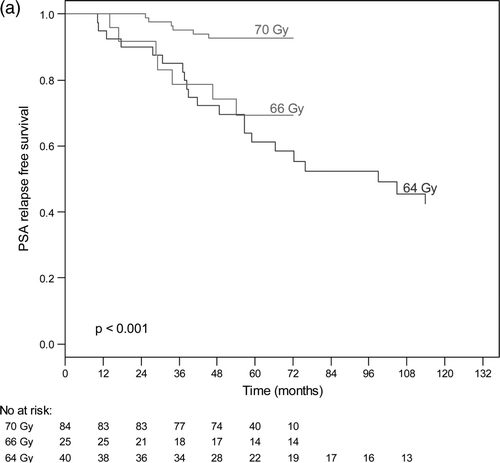
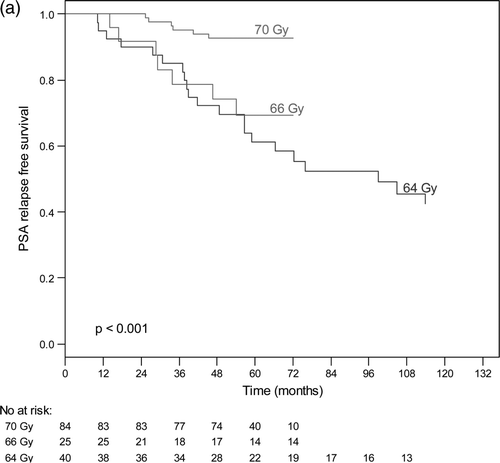
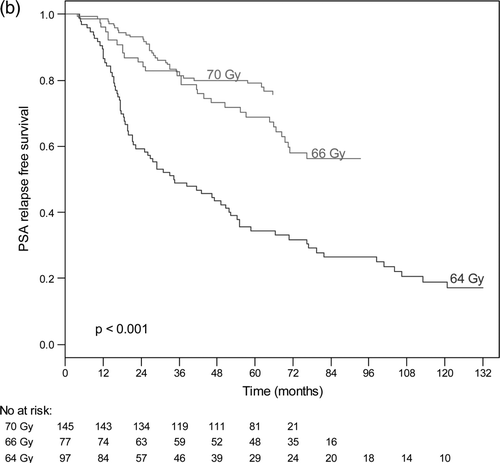
The actuarial 5-years bNED was 100%, 80% and 63% for patients in the low risk group, intermediate risk group and high risk group respectively (). For low risk tumours, the 5-year bNED rate was 100% for all radiation doses. The actuarial bNED rates for intermediate and high-risk patients are shown in and , according to dose. For intermediate risk tumours, the 5-year bNED rate for patients who received a radiation dose of 70 Gy, 66 Gy or 64 Gy was 92%, 69% and 61%, respectively (p < 0.001) (). For high-risk tumours, the 5-year bNED rate for patients who received radiation dose 70 Gy, 66 Gy and 64 Gy was 79%, 69% and 34%, respectively (p < 0.001) (). Thus treatment to 70 Gy significantly improved the PSA relapse-free outcome of intermediate and high-risk patients. a shows the results of a Cox proportional hazard multivariate analysis of factor affecting PSA relapse. Risk group, radiation doses and hormone therapy were significant independent predictors of PSA failure.
Table II. Results from multivariate Cox regression, assessing influence of different parameters on: a) PSA recurrence, b) cancer specific survival, c) overall survival.
Cancer specific survival
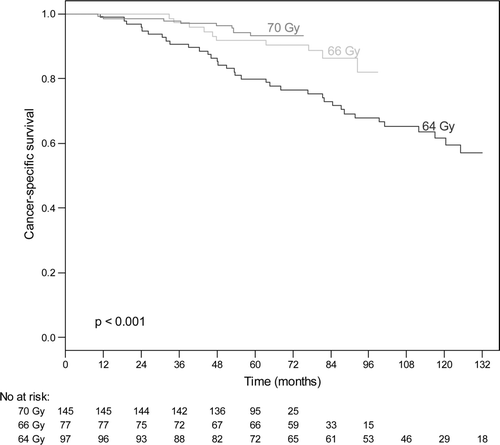
The 5-year and 10-year CSS rate for all cases were 92% (95% CI: 89–94%), and 75%, respectively. For low risk tumours, the 5-year CSS rate was 100% for all dose levels. For intermediate risk prostate tumours, the 5-year CSS rate for patients who received radiation doses of 70 Gy, 66 Gy or 64 Gy was 100%, 100% and 92%, respectively (p = 0.09). In the high risk group, the 5-year CSS rate for patients who received radiation doses of 70 Gy, 66 Gy and 64 Gy was 93%, 92% and 80%, respectively (p < 0.001) (). b shows the results of Cox proportional hazards multivariate analysis of factors affecting CSS. Risk group and radiation dose were significant independent predictors of CSS, while there was a trend (p = 0.06) for hormone therapy.
Overall survival
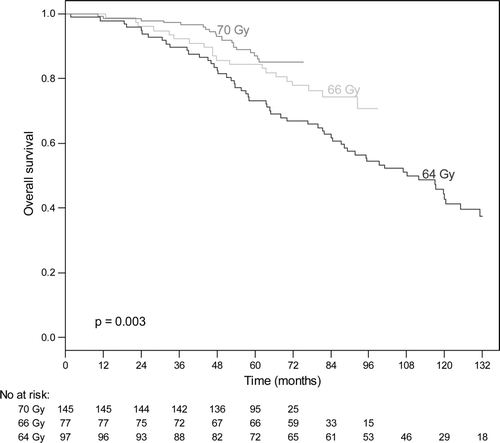
The 5-year OS rate for the entire group was 85% (95% CI: 81–88%), at 10 years 54%. For low risk tumours, the 5-years OS rate was 77%. For intermediate risk tumours, the 5-years OS rate for patients who received radiation doses of 70 Gy, 66 Gy or 64 Gy was 95%, 88% and 87%, respectively (p = 0.28). For high risk tumours, the 5-year OS rate for patients who received radiation doses of 70 Gy, 66 Gy and 64 Gy was 87%, 84% and 73%, respectively (p = 0.003) (). c shows the result of Cox proportional hazards multivariate analysis of factor affecting OS. Risk group and radiation doses were significant independent factors affecting OS, although the low and intermediate risk groups had not significantly different OS.
Discussion
The present study revealed a radiation dose response for both intermediate risk and high risk patients with localised adenocarcinoma of the prostate treated with radiation, with or without hormone treatment. A limitation of consecutive series may be change in selection criteria and treatment with time. Earlier diagnosis due to more frequent PSA testing may indicate a time trend towards improved outcome Citation[14] but we could not show such a relationship with time in our cohorts Citation[5].
For low risk patients, there are several therapy options. The low number in our cohort does not allow general conclusions regarding dose in this group. We did not recommend hormone therapy to all patients, but the referring urologists considered RT to be less effective than surgery and started with hormone therapy, a trend also observed in USA during the 1990s Citation[15]. The preference for hormone therapy was probably also influenced by the SPCG 7 (Scandinavian Prostate Cancer Group) trial which was conducted at that time, using hormones for all patients and randomizing patients to radiation or control Citation[16]. At the time of the current study hormone therapy was therefore more widely used across all stages and grades than the present more evidence-based approach where hormone therapy is limited to locally advanced prostate tumours Citation[17].
The present study demonstrated a dose-dependent improvement in both bNED and CSS in high risk patients and also improvement in bNED in intermediate risk patients that with longer follow-up may improve survival. Our findings were confirmed in a recent phase III study testing a dose of 64 Gy vs. 74 Gy in conjunction with initial androgen suppression, where bNED was significantly higher in the 74 Gy group Citation[18].
A randomized controlled trial by Zietman and colleagues similarly showed that increasing the dose from 70.2 to 79.2 Gy, improved bNED both for low risk and intermediate risk patients Citation[19]. In the study by Pollack and colleagues, increasing the radiation dose from 70 Gy to 78 Gy lead to a further substantial improvement in bNED rates for patients with intermediate to high risk prostate cancer, and there was an intriguing decrease in distant metastases seen with the higher doses Citation[20]. A recent Dutch phase III trial have also reported a dose response for bNED in intermediate and high risk patients Citation[21], while Zelefsky and co-workers Citation[22] reported that radiation dose was the most powerful variable to improve bNED in all prognostic risk group. Patients with a large prostate volume were given a 3-months course of neoadjuvant complete androgen deprivation to decrease prostate size in this study. The authors concluded that even for the more favourable tumours, conventional radiation doses of 65–70 Gy alone are ineffective for local tumour control. The study indicates that doses on the order of 81 Gy may be necessary to achieve maximal local cure. Other authors have also reported that radiation doses in the range 74–81 Gy improve tumour control in the high risk group Citation[23], Citation[24].
In our cohort of patients, neoadjuvant and adjuvant HT was used in 402 of the 494 (81%) patients, which probably has contributed to improve the outcome Citation[5], however only 9% of the 402 patients had long-term androgen deprivation. Randomized trials of adjuvant androgen deprivation therapy combined with external beam radiation therapy for patients with locally advanced tumours have demonstrated a clear survival benefit compared with radiation therapy alone Citation[17], Citation25–28. Recent studies have demonstrated that the addition of 6 months of androgen deprivation given before and during radiotherapy (66–70 Gy) confers an overall survival benefit for patients with clinically localized prostate cancer Citation[26], Citation[27]. The long-term results of RTOG 8610 demonstrate that addition of 4 months of androgen deprivation to EBRT (65–70 Gy) in men with locally advanced prostate cancer has impact on CSS, distant metastases, disease-free survival and bNED Citation[28]. The study of Bolla et al. Citation[17] showed that immediate androgen suppression with a luteinising-hormone releasing hormone analogue given during and for 3 years after external irradiation (70 Gy) further improves disease-free and overall survival of patients with locally advanced prostate cancer. Also, high risk patients have been shown to have better tumour control after adjuvant androgen deprivation, whereas low risk patients do not appear to substantially benefit from adjuvant androgen deprivation after 8 years follow-up Citation[29]. Whether androgen deprivation may become unnecessary as suggested by the RTOG 9407 study Citation[30] when higher dose levels are administered remains an open question. The EORTC Phase III trial 22961 compared short (6 months) versus long (3 years) term concomitant and adjuvant androgen deprivation with EBRT (70 Gy) in patients with locally advanced prostate cancer. Long-term hormone therapy was more effective than short-term androgen suppression in these locally advanced tumours and is now considered the standard of care for such patients Citation[31]. Results from the SPCG 7 randomized phase III trial has demonstrated a 10% absolute survival benefit after a median follow-up of 7.5 years, from addition of EBRT to long-term HT, in patients with locally advanced prostate cancer Citation[16]. The absolute difference in bNED at 7 years was 53.5%, indicating that with longer follow-up the survival benefit will further increase. The Early Prostate Cancer Trial support that hormone therapy should not be used as the only curative treatment except in the elderly or specially selected cases Citation[32].
Conclusion
We report that a higher radiation dose (70 Gy) improved the outcome for bNED and CSS in high risk prostate cancer patients, in the intermediate risk group the effect was only demonstrated for bNED.
Acknowledgements
This study was supported by generous grants from the Norwegian Cancer Society. Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Cancer Registry of Norway. Cancer in Norway 2007. OsloNorway, 2008.
- Martinez AA, Gonzalez JA, Chung AK, et al. A comparison of external beam radiation therapy versus radical prostatectomy for patients with low risk prostate carcinoma diagnosed, staged, and treated at a single institution. Cancer 2000; 88: 425–32
- D'Amico AV, Whittington R, Kaplan I, et al. Equivalent 5-year bNED in select prostate cancer patients managed with surgery or radiation therapy despite exclusion of the seminal vesicles from the CTV. Int J Radiat Oncol Biol Phys 1997; 39: 335–40
- Bill-Axelso A, Holmberg L, Filen F, et al. Radical prostatectomy versus watchful waiting in localized prostate cancer: The Scandinavian prostate cancer group-4 randomized trial. J Natl Cancer Inst 2008; 100: 1144–54
- Karlsdottir A, Muren LP, Wentzel-Larsen T, et al. Radiation dose escalation combined with hormone therapy improves outcome in localised prostate cancer. Acta Oncol 2006; 45: 454–62
- Hermanek, P, Sobin, LH, TNM classification of malignant tumours. 4th edn. International Union Against Cancer (UICC). 1992, Berlin, Heidelberg, New York: Springer Verlag.
- Mostofi, FK, Sesterhenn, IA, Sobin, LH. Histological typing of prostate tumours. Geneva: World Health Organization; 1980.
- Mellinger GT, Gleason D, Bailar J. The histology and prognosis of prostatic cancer. J Urology 1967; 97: 331–8
- Van der Kwast TH, Roobol MJ, Wildhagen MF, et al. Consistency of prostate cancer grading results in screened populations across Europe. BJU Int 2003; 92(Suppl 2)88–91
- Karlsdottir A, Johannessen DC, Muren LP, et al. Acute morbidity related to treatment volume during 3D-conformal radiation therapy for prostate cancer. Radiother Oncol 2004; 71: 43–53
- Roach III M, Hanks G, Thames H, Jr., et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 2006; 65: 965–74
- Karlsdottir A, Muren LP, Wentzel-Larsen T, et al. Late gastrointestinal morbidity after three-dimensional conformal radiation therapy for prostate cancer fades with time in contrast to genitourinary morbidity. Int J Radiat Oncol Biol Phys 2008; 70: 1478–86
- Altman, D. Practical statistics for medical research. London, New York: Chapman and Hall; 1991. p 611.
- Kupelian P, Thames H, Levy L, et al. Year of treatment as independent predictor of relapse-free survival in patients with localized prostate cancer treated with definitive radiotherapy in the PSA era. Int J Radiat Oncol Biol Phys 2005; 63: 795–9
- Shahinian VB, Kuo YF, Freeman JL, et al. Increasing use of gonadotropin-releasing hormone agonists for the treatment of localized prostate carcinoma. Cancer 2005; 103: 1615–24
- Widmark A, Klepp O, Solberg A, et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): An open randomised phase III trial. Lancet 2009; 373: 301–8
- Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): A phase III randomised trial. Lancet 2002; 360: 103–8
- Dearnaley DP, Sydes MR, Graham JD, et al. Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: First results from the MRC RT01 randomised controlled trial. Lancet Oncol 2007; 8: 475–87
- Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: A randomized controlled trial. JAMA 2005; 294: 1233–9
- Pollack A, Zagars GK, Starkschall G, et al. Prostate cancer radiation dose response: Results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys 2002; 53: 1097–105
- Peeters ST, Heemsbergen WD, Koper PC, et al. Dose-response in radiotherapy for localized prostate cancer: Results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol 2006; 24: 1990–6
- Zelefsky MJ, Fuks Z, Hunt M, et al. High dose radiation delivered by intensity modulated conformal radiotherapy improves the outcome of localized prostate cancer. J Urol 2001; 166: 876–81
- Klotz LH. Active surveillance for good risk prostate cancer: Rationale, method, and results. Can J Urol 2005; 12(Suppl 2)21–4
- Warlick C, Trock BJ, Landis P, et al. Delayed versus immediate surgical intervention and prostate cancer outcome. J Natl Cancer Inst 2006; 98: 355–7
- Bolla M, Gonzalez D, Warde P, et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med 1997; 337: 295–300
- D'Amico AV, Manola J, Loffredo M, et al. 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: A randomized controlled trial. JAMA 2004; 292: 821–7
- Denham JW, Steigler A, Lamb DS, et al. Short-term androgen deprivation and radiotherapy for locally advanced prostate cancer: Results from the Trans-Tasman Radiation Oncology Group 96.01 randomised controlled trial. Lancet Oncol 2005; 6: 841–50
- Roach M, 3rd, Bae K, Speight J, et al. Short-term neoadjuvant androgen deprivation therapy and external-beam radiotherapy for locally advanced prostate cancer: Long-term results of RTOG 8610. J Clin Oncol 2008; 26: 585–91
- Zeliadt SB, Potosky AL, Penson DF, et al. Survival benefit associated with adjuvant androgen deprivation therapy combined with radiotherapy for high- and low-risk patients with nonmetastatic prostate cancer. Int J Radiat Oncol Biol Phys 2006; 66: 395–402
- Valicenti R, Bae K, Michalski J, et al. Does adjuvant hormonal therapy improve freedom from biochemical relapse in prostate cancer patients receiving dose-escalated radion therapy? An analysis of RTOG 94-06. Int J Radiat Oncol Biol Phys 2007; 69: s171
- Bolla M, van Tiehoven G, de Reijke TM, et al. Concomitant and adjuvant androgen deprivation (ADT) with external beam irradiation (RT) for locally advanced prostate cancer: 6 months versus 3 years ADT – Results of the randomized EORTC Phase III trial 22961. J Clin Oncol 2007; 25: s238
- McLeod DG, Iversen P, See WA, et al. Bicalutamide 150 mg plus standard care vs standard care alone for early prostate cancer. BJU Int 2006; 97: 247–54