Increasing incidence of cancer, improving survival rates, and extended use of platinum-containing chemotherapy all add to the rising prevalence of platinum-induced neuropathy. The importance of systematic survivorship studies is generally acknowledged and it has been revealed that 10–15% of testicular cancer survivors suffer from paresthesias after a median observation time of 10 years Citation[1], Citation[2]. Anticipated treatment-induced toxicities and quality of life (QoL) issues are nowadays routinely integrated into clinical decision making Citation[3]. The impact of platinum-induced neuropathy does not only affect QoL, since treatment pauses, dose reductions or even discontinuation of chemotherapy due to increasing neuropathy may in fact reduce the chances of survival.
Therefore, we consider the approach of Brouwers et al., published in this issue of Acta Oncologica Citation[4], to assess the prevalence of persistent neuropathy after cisplatin or oxaliplatin containing chemotherapy and potential risk factors as timely and important.
Cisplatin's neurotoxic effects increase often over weeks after application and are usually limited to sensory functions- and do usually not impair motor functions Citation[5]. Oxaliplatin-induced neurotoxicity occurs immediately after infusion, and comprises in addition to paresthesias muscle spasms and fasciculations, which typically are precipitated by cold-exposure. Apparently, nerves are hyper-excitable, a finding which has been related to interference of oxaliplatin with the function of voltage-gated sodium channels function Citation[6]. Acute oxaliplatin neurotoxicity is experienced only during the first days after application, but at cumulative doses of 800 mg/m2 the risk of long-term paresthesias increases, particularly in the feet. Although axonal damages have been related to platinum-containing drugs, the principal pathophysiologic correlate of long-term sensory neuropathy consists of dorsal nerve ganglion degeneration where these drugs accumulate Citation7–9.
The inter-individual variation of platinum-induced neurotoxicity is remarkable. For both agents genetic polymorphisms of glutathione S-transferase have been demonstrated to explain some of individual susceptibility Citation10–12. There are probably many more genetic variants determining the individual patient's location in the broad spectrum between resilience and vulnerability to platinum-containing chemotherapy Citation[13]. Only well-designed large clinical studies, ideally containing all patients undergoing chemotherapy, will allow elucidation of these complex relationships. Whereas genotyping may be performed by external centers of especial expertise – if a blood sample has been collected-, phenotyping relies on clinicians and might represent the most critical limitation of future association studies.
Phenotyping may be performed by asking patients to report their symptoms, i.e. subjective neuropathy. Alternatively, neurophysiologic measurements may be applied, e.g. vibration threshold testing. Unfortunately, the correlation between vibration threshold testing and scales for the assessment of chemotherapy-induced neuropathy is only moderate Citation[14].
Therefore, one may have to choose between reproducibility and exactness, i.e. objective measurements, and subjective symptoms which may have a stronger impact on our patients QoL. Jensen et al. illustrated the relation of objectivity, i.e. specificity, and subjectivity, i.e. relevance () Citation[15].
Figure 1. Illustration of the cause effect and the trade off between relevance and specificity of different measures of side effects.
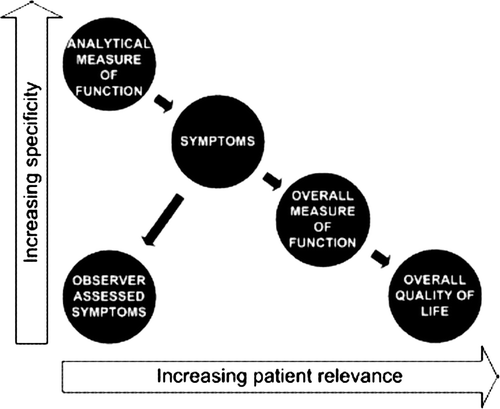
Thereby, treatment-induced neuropathy might be considered as an area defined by subjective and objective findings. Accordingly, the approach of Brouwers et al. Citation[4] to comprehensively assess objective neurophysiological outcomes as well as functional ones as gait and Romberg's test in addition to patient reported outcomes should prove suitable for the exploration of platinum-induced neuropathy.
Generation of multiple end-points, however, necessitates multiple statistical tests, which should be corrected for by increased p-values for statistical significance. This may pose a challenge for extended studies by this group. Nevertheless, if a given risk factor would be associated with both subjective, objective, and possibly functional examinations, its relevance would be more convincing than any single statistically significant correlation.
Ideally, the test armamentarium would comprise assessment of the density of epidermal nerve fibers by skin biopsy Citation[16], as well as testing of the function of autonomic fibers by quantitative sudomotor axon reflex test (QSART) Citation[17].
The appropriateness of subjective tests depends on demonstration of their validity by psychometric analyses. The questions used in the study by Brouwers et al. were extracted from other established questionnaires and further validation beyond a satisfactory Cronbach's alpha coefficient are mandatory Citation[4].
Intriguingly, self-reported item scores on neuropathy are influenced by personality traits. Grov et al. Citation[18], report in this issue of Acta Oncologica on the association between a tendency towards neuroticism and an impressively long list of various outcomes reported by 1 428 testicular cancer survivors. The authors introduced the trait of ‘neuroticism’ to represent the person's tendency to feel nervous and worrying rather than feeling calm and safe. Of particular interest might be the finding that typical cisplatin-induced toxicities like paresthesias, Raynaud's phenomena, hearing impairment, and tinnitus were significantly more frequently reported by the 176 (12%) testicular cancer survivors who were considered as ‘neurotic’ compared the majority of more relaxed and confident ones. Unfortunately, this study did not contain objective measurements such that the admittedly unlikely hypothesis of a possibly increased and not only a perceived vulnerability to chemotherapy induced toxicities can not be rejected. Thereby, in an ideal world, the personality of participants should be assessed as well as subjective and objective parameters enabling comprehensive multivariate analyses.
The article of Brouwers et al. is easy to criticize as the low number of patients precludes extraction of clear messages for clinical practice. Such small patient samples are prone to several kinds of bias and confounding. However, it is not the aim of this pilot study to avoid these errors but rather to identify them: Lung cancer patients, receiving cisplatin might have some degree of neuropathy due to smoking and not exclusively due to chemotherapy. Alcohol consumption is associated with smoking and might represent a confounding factor. However, exclusion of patients with such competing risk factors prevents estimation of their respective impact on neuropathy and would leave a highly selected sample of patients which probably would not be representative for cancer patients in general. For subsequent series the authors should however comment on the application of radiotherapy as this treatment very well may cause neurologic complications, especially since platinated substances are known to function as radiosensitizers Citation[19], Citation[20].
Prevention of platinum-induced neurotoxicity by protective agents is controversial since beneficial effects have not been demonstrated unequivocally, yet. Further, concerns of a possibly diminished anti-tumor effect hamper the introduction of protective agents in humans. Recent ASCO guidelines on that topic did not recommend amifostine for prevention of chemotherapy induced neuro- or ototoxicity Citation[21]. Treatment of platinum-induced neuropathy is difficult and may require several drugs like anticonvulsants, tricyclic antidepressants, and in particularly severe cases morphine Citation[22].
In our view there are more questions than answers and more uncertainties than knowledge concerning the various aspects of platinum-induced neuropathy like incidence, prevention, associated risk factors including genetic susceptibility. Therefore explorative studies as that planned by Brouwers et al. represent a valuable contribution to the elucidation of platinum-induced neuropathy and, hopefully, its prevention.
References
- Fossa SD. Long-term sequelae after cancer therapy – Survivorship after treatment for testicular cancer. Acta Oncol 2004; 43: 134–141
- Nord C, Ganz PA, Aziz N, Fossa SD. Follow-up of long-term cancer survivors in the Nordic countries. Acta Oncol 2007; 46: 433–440
- O'Dwyer PJ, Eckhardt SG, Haller DG, Tepper J, Ahnen D, Hamilton S, et al. Priorities in colorectal cancer research: Recommendations from the Gastrointestinal Scientific Leadership Council of the Coalition of Cancer Cooperative Groups. J Clin Oncol 2007; 25: 2313–2321
- Brouwers, E, Huitema, A, Boogerd, W, Beijnen, J, Schellens, J. Persistent neuropathy after treatment with cisplatin and oxaliplatin. Acta Oncol 2009, (in press).
- Hausheer FH, Schilsky RL, Bain S, Berghorn EJ, Lieberman F. Diagnosis, management, and evaluation of chemotherapy-induced peripheral neuropathy. Semin Oncol 2006; 33: 15–49
- Webster RG, Brain KL, Wilson RH, Grem JL, Vincent A. Oxaliplatin induces hyperexcitability at motor and autonomic neuromuscular junctions through effects on voltage-gated sodium channels. Br J Pharmacol 2005; 146: 1027–1039
- Meijer C, de Vries EGE, Marmiroli P, Tredici G, Frattola L, Cavaletti G. Cisplatin-induced DNA-platination in experimental dorsal root ganglia neuronopathy. Neurotoxicology 1999; 20: 883–887
- McKeage MJ, Hsu T, Screnci D, Haddad G, Baguley BC. Nucleolar damage correlates with neurotoxicity induced by different platinum drugs. Br J Cancer 2001; 85: 1219–1225
- McDonald ES, Randon KR, Knight A, Windebank AJ. Cisplatin preferentially binds to DNA in dorsal root ganglion neurons in vitro and in vivo: A potential mechanism for neurotoxicity 195. Neurobiol Dis 2005; 18: 305–313
- Lecomte T, Landi B, Beaune P, Laurent-Puig P, Loriot MA. Glutathione S-transferase P1 polymorphism (Ile(105)Val) predicts cumulative neuropathy in patients receiving oxaliplatin-based chemotherapy. Clin Cancer Res 2006; 12: 3050–3056
- Oldenburg J, Kraggerud SM, Cvancarova M, Lothe RA, Fossa SD. Cisplatin-induced long-term hearing impairment is associated with specific glutathione S-transferase genotypes in testicular cancer survivors. J Clin Oncol 2007; 25: 708–714
- Oldenburg J, Kraggerud SM, Brydoy M, Cvancarova M, Lothe RA, Fossa SD. Association between long-term neuro-toxicities in testicular cancer survivors and polymorphisms in glutathione-s-transferase-P1 and -M1, a retrospective cross sectional study. J Transl Med 2007; 5: 70
- Shukla SJ, Duan S, Badner JA, Wu XL, Dolan ME. Susceptibility loci involved in cisplatin-induced cytotoxicity and apoptosis. Pharmacogenet Genomics 2008; 18: 253–262
- Cavaletti G, Bogliun G, Marzorati L, Zincone A, Piatti M, Colombo N, et al. Grading of chemotherapy-induced peripheral neurotoxicity using the Total Neuropathy Scale. Neurology 2003; 61: 1297–1300
- Jensen K. Measuring side effects after radiotherapy for pharynx cancer. Acta Oncol 2007; 46: 1051–1063
- Ebenezer GJ, Hauer P, Gibbons C, McArthur JC, Polydefkis M. Assessment of epidermal nerve fibers: A new diagnostic and predictive tool for peripheral neuropathies. J Neuropathol Exp Neurol 2007; 66: 1059–1073
- Gibbons CH, Illigens BM, Centi J, Freeman R. QDIRT: Quantitative direct and indirect test of sudomotor function. Neurology 2008; 70: 2299–2304
- Grov, EK, Dahl, AA, Fossa, SD. Neuroticism. Acta Oncol 2009, (in press).
- Knap MM, Bentzen SM, Overgaard J. Late neurological complications after irradiation of malignant tumors of the testis. Acta Oncol 2007; 46: 497–503
- Brydoy M, Storstein A, Dahl O. Transient neurological adverse effects following low dose radiation therapy for early stage testicular seminoma. Radiother Oncol 2007; 82: 137–144
- Hensley ML, Hagerty KL, Kewalramani T, Green DM, Meropol NJ, Wasserman TH, et al. American Society of Clinical 2008 Oncology clinical practice guideline update: Use of chemotherapy and radiation therapy protectants. J Clin Oncol 2009; 27: 127–145
- Kaley, TJ, Deangelis, LM. Therapy of chemotherapy-induced peripheral neuropathy. Br J Haematol 2009.