Abstract
Background: Women irradiated for left-sided breast cancer (BC) have an increased risk of coronary artery disease compared to women with right-sided BC. We describe the distribution of radiation dose in segments of coronary arteries in women receiving adjuvant radiotherapy (RT) for left- or right-sided BC.
Material and methods: Fifteen women with BC, seven left-sided and eight right-sided, who had received three-dimensional conformal radiotherapy (3DCRT), constituted the study base. The heart and the segments of the coronary arteries were defined as separate organs at risk (OAR), and the mean and maximum radiation doses were calculated for each OAR.
Results: In women with left-sided BC, irrespective of if regional lymph node RT was given or not, maximum dose in mid and distal left anterior descending artery (mdLAD) was approximately 50 Gy in 6/7 patients, whereas women with right-sided BC mainly received low doses of radiation. In women with left-sided BC, 6/7 patients had substantially higher mean dose to the distal LAD than to the heart, ranging from 30 to 55 Gy and 3 to13 Gy, respectively.
Conclusion: We found a pronounced difference of radiation dose distribution in the coronary arteries between women with left- and right-sided BC. Women with left-sided BC had almost full treatment dose in parts of mdLAD, regardless of if regional lymph node irradiation was given or not, while women with right-sided BC mainly received low doses to the coronary arteries.
Meta-analyses of randomized clinical trials show a benefit of adjuvant radiotherapy (RT) in breast cancer (BC) in terms of reducing local recurrences and BC deaths [Citation1–3]. However, long-term follow-up has revealed an excess mortality from heart disease after RT [Citation3]. RT in the 1970s and 1980s is associated with a higher mortality due to ischemic heart disease (IHD) in left-sided BC compared to right-sided [Citation4], but conflicting results have been published on more modern RT [Citation5,Citation6]. The pathophysiology of radiation-induced heart disease involves microangiopathy of the small vessels as well as macroangiopathy of the coronary arteries [Citation7], resulting in fibrosis of the myocardium, coronary artery disease and eventually IHD. These multiple targets of radiation raise the question which the most critical cardiac structures are – the myocardium or the coronary arteries?
Hitherto, the whole heart has been considered as ‘one’ organ at risk (OAR) and tolerance doses have been established for pericarditis [Citation8] and cardiovascular mortality [Citation9]. However, the different anatomical structures in the heart may have different radiation tolerance, illustrated by the study of McGale et al. [Citation10]. In a comparison between women with irradiated left- and right-sided BC, an increase of angina pectoris and myocardial infarction was noticed, reflecting coronary artery disease. On the contrary, the same comparison showed no increase in heart failure or myocardial diseases. These results support macroangiopathy rather than microangiopathy being the most detrimental pathogenetic mechanism of radiation-induced IHD in BC. However, the study by Darby et al. found a dose-dependent linear increase of IHD events with a stronger correlation to heart mean dose than to left anterior descending artery (LAD) mean dose [Citation11].
Our recent study showed that coronary artery stenosis was more frequent within the geometric projection of the radiation fields, in contrast to other locations of the heart [Citation12]. The aim of this study was to describe the distribution of radiation dose in segments of coronary arteries in women with left- and right-sided BC who have received three-dimensional conformal radiotherapy (3DCRT) and to study the relation of the dose to the postulated radiation hotspot areas of the ventral segments of the coronary arteries defined in our former study [Citation12]. We also report the distribution of coronary artery stenoses.
Material and methods
Patient selection
The women in our study were selected from a Swedish cohort of 8190 women with a first BC diagnosed from 1970 to 2003. The cohort is described in detail previously [Citation12,Citation13]. To find women who had been referred to a coronary angiography after BC diagnosis, the cohort was linked to two regional registers of coronary angiography in the hospitals of Uppsala and Falun, covering the time period from 1990 to 2004. The linkage yielded 199 eligible women, which was the study base of our former study [Citation12]. The use of 3DCRT in BC started in the 1990s and 23 women in the study base had received 3DCRT in the Radiotherapy Departments of Uppsala and Gävle. Three women were irradiated after the angiography, leading to exclusion. Due to missing information from computed tomography (CT) studies, five other women were excluded, leaving in total 15 eligible women. The time to angiography was defined as the time period from the date of RT termination to the date of angiography.
Data collection
The medical records were the source of information for breast tumor characteristics and details of BC treatments. The RT records were reviewed for classification of target areas: remaining breast tissue after breast conserving surgery, regional lymph nodes (reg LN) in the axilla, internal mammary chain (IMC), and supraclavicular (SCL) area. Information about adjuvant endocrine treatment, chemotherapy, and recurrences was abstracted from the medical records.
Coronary angiography
The coronary angiograms from Uppsala and Falun were reviewed by one radiologist in each hospital. The right coronary artery (RCA), the left main coronary artery (LMCA), the LAD, and the left circumflex artery (LCX), were further divided into 18 segments (Figure S1 in Supplementary material, available online at http://www.informahealthcare.com) [Citation14]. The segments were graded according to a five-grade scale of stenosis, where grade 0 indicated a normal segment without any atheromatosis or stenosis; grade 1 light atheromatosis; grade 2–4, increasing grade of stenosis; and grade 5, occlusion of the segment of the vessel [Citation14]. Grade 3–5 stenosis was considered clinically significant, referred to as significant stenosis.
3D conformal radiotherapy
The individual CT study of each patient was retrieved in the treatment planning system Helax-TMS®. The CT scans were not contrast enhanced. CT slices measured 8 or 16 mm. The original target definition from the treatment session was used. Eleven patients received tangential RT with opposed photon beams to cover the targets (Tang in Table S1 in Supplementary material, available online at http://www.informahealthcare.com). The treatment plans were individually optimized with beam angles, wedges, collimator angles, and multileaf collimators (MLC). The patients irradiated to the breast only, were treated using the technique of aligned posterior border. The patients irradiated also to reg LN with the tangential technique, were treated using MLC. One patient irradiated to the breast and reg LN was treated with ‘the double angle technique’ (Double Tang in Table S1), a tangential radiation technique with a steeper gantry angle in the superior part of the target to cover the SCL and IMC and a flatter gantry angle in the inferior part of the target to cover the breast and simultaneously minimize the radiation dose to the defined OARs, i.e. the lung and the heart. Three patients irradiated to the breast and reg LN were treated with a technique developed in the department, using mixed electron-photon beams conformed with a MLC (Mixed in Table S1), described in detail by Jansson et al. [Citation15].
Two of the authors defined in consensus the following segments of the coronary arteries as separate OARs: segment 1, 2, 3, 5, 6, 7, and 8 (Figure S1) [Citation14]. Segments 1 + 2, corresponding to the proximal RCA, are hereafter referred as prox RCA, and segments 7 + 8, corresponding to the mid and distal LAD, are hereafter referred as mdLAD. Reliable anatomical cardiac landmarks, including the anterior interventricular, left atrioventricular and right atrioventricular grooves, were used to define the coronary artery positions [Citation16]. A margin of 2–3 mm was added to each OAR to allow for uncertainties regarding the exact position of the coronary artery and to assure that the coronary artery were within the OAR. The whole heart was also defined as an OAR.
The previously individually optimized treatment plans were used and dose-volume histograms were generated for the defined OARs of segment 1, 2, 3, 5, 6, 7, 8, and the heart. For each OAR, mean and maximum radiation doses were assessed for individual patients. For the heart as an OAR, the volumes receiving 40 Gy (Vheart 40 Gy) and 20 Gy (Vheart 20 Gy) were calculated. The dose distributions in the treatment planning system were calculated using a pencil beam algorithm.
Results
The patient characteristics are described in Table S1. In total, 15 women with BC, seven cases of left- and eight cases of right-sided BC, were studied. Eight women were diagnosed with BC stage II, four women had BC stage I and for three women stage information from the axillary surgery was missing (Table S1). The BCs were diagnosed between 1993 and 2002. Mean age at diagnosis of BC was 58.9 years (range 46–70 years) and mean follow-up period between termination of RT and coronary angiography was 3.8 years (range 0.3–8.2 years).
Eight women received RT to the breast and reg LN: the axilla, SCL, and IMC included. Two women were irradiated to the breast and axilla, and five women received RT to the breast without reg LN. Fourteen women were treated with 25–28 radiation fractions of 2.0 Gy to the targets five days each week, to a total dose of 50–56 Gy. One woman, Patient 9, received 16 fractions of 2.0 Gy and 10 fractions of 2.1 Gy, to a total dose of 53 Gy. Five women received adjuvant chemotherapy (chemotherapy regimens shown in Table S1). Six women used adjuvant endocrine therapy during the study, all of them tamoxifen. One woman, Patient 7, started with tamoxifen and changed endocrine treatment after seven months to anastrozole. No women experienced local or distant recurrences during the follow-up.
and show the distribution of radiation doses. All women with right-sided BC had low mean doses to the heart in the range of 1–3 Gy. Women with left-sided BC had higher mean doses to the heart in the range of 3–13 Gy. Mean doses to mdLAD and in particular segment 8 (corresponding to distal LAD), ranging from 18 to 55 Gy, were considerably higher than mean heart doses in women with left-sided BC, reflecting high doses in small volumes. In three of the women, mean doses to prox RCA and in particular segment 2 were substantially higher than mean doses to the heart, ranging from 21 to 33 Gy and 3 to 12 Gy, respectively. In general, the radiation doses were higher in the ventral located segments 1 + 2 and segments 7 + 8, in comparison to the doses of the dorsal adjacent segment 3 and segment 6, respectively ( and ).
Figure 1. Segment-wise distribution of mean maximal radiation doses, of seven left- and eight right-sided women with breast cancer (BC). Gy: Gray; LMCA: left main coronary artery; LAD: left anterior descending artery; RCA: right coronary artery.
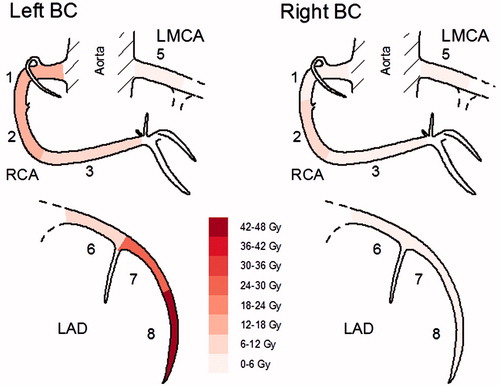
Table 1. Radiation doses to targets, heart, and segments of coronary arteries.
Three of the women with left-sided BC receiving RT to the breast and reg LN had significant volumes of the heart (>10%) irradiated with doses exceeding 20 Gy (Table S1). The distribution of radiation dose in the segments of the coronary arteries differed markedly between left- and right-sided BC ( and ). Irradiation of left-sided BC gave a substantially higher radiation dose in mdLAD compared to right-sided BC, with doses in segment 8 approaching the target dose of 50–56 Gy. The two women with left-sided BC receiving RT to the breast without reg LN irradiation had doses in segment 8 as high as the women irradiated also to reg LN.
Women with right-sided BC generally received low coronary artery mean doses in the range of 1–5 Gy. The only exception was Patient 9, a woman where the irradiation of the right IMC contributed to the maximum dose of 45 Gy in segment 2. Two of the women with left-sided BC, Patients 1 and 3, also received substantial maximum doses of 38–39 Gy in prox RCA, both of which were irradiated to the left IMC. These three women were irradiated in the early period of the study when the IMC target included the ipsilateral intercostal spaces 1–5. After 1995, the target definition of IMC changed to include only intercostal spaces 1–3 with the purpose of lowering the heart dose, and thereafter all women irradiated to the IMC had low radiation doses in prox RCA with maximum doses ranging from 3 to 6 Gy (Table S1).
The majority of the coronary segments studied had no stenoses (Table S1). In four patients, seven segments had clinical significant stenoses. In Patient 4, a significant stenosis was located in a hotspot area of radiation dose, whereas in the other three patients the significant stenoses were mostly located in areas with low exposition of radiation.
Discussion
We found a marked difference of radiation dose distribution in mdLAD between women with left- and right-sided BC. The majority of the women with left-sided BC had high mean radiation doses in distal LAD, in the range of 30–55 Gy, whereas the women with right-sided BC mostly were exposed to low radiation doses. Maximum doses close to full target doses in distal LAD were noticed in patients with left-sided tangential RT of the breast, regardless of reg LN irradiation. There was a radiation dose gradient with higher doses in the most ventral coronary segments and lower doses in segments located more dorsally.
In contrast to previous studies of older RT techniques [Citation11,Citation17] requiring dose estimations of representative average patients, this study is based on detailed information of the individual patient radiation dose in different parts of the heart and coronary arteries, as all the patients have been treated with 3DCRT. In a previous investigation by our group not based on individual dose plans, hotspot areas for radiation dose of prox RCA and mdLAD were postulated [Citation12]. This was confirmed in the present study. Our result is in accordance with the study by Taylor et al., showing an average maximum radiation dose of 35.2 Gy in the LAD in women receiving left-sided tangential RT for BC [Citation18]. The LAD average mean dose was considerably lower [Citation18], reflecting the anatomy of the LAD in relation to the radiation fields and the fact that small volumes of the artery, i.e. segments may receive high doses, albeit the mean dose of the artery being within tolerance limits.
The study has some shortcomings. Small volumes, like segments of coronary arteries, and thicker CT slices than today, implicate fewer points for dose calculations and poorer accuracy in dose evaluation. Low doses of scattered and transmitted radiation within the patient and through field shaping devices, contributes to an additional uncertainty in estimation of delivered dose to areas outside the clinical beam of approximately 1–2% relative to the dose in target regions [Citation19]. However, these caveats do not influence the main result in any significant way, as the differences between radiation doses in left- and right-sided BC and between segments are obvious.
The study was initially designed to also explore eventual strong relationship between radiation dose and coronary artery stenosis in specific coronary segments with known dose. However, we found considerably fewer women than we expected that had both angiograms and were irradiated with 3DCRT. Furthermore, the interval between RT and the angiogram was generally short in terms of looking for radiation-induced lesions. Coronary artery disease is a multifactorial and common disease, and also in an irradiated BC population the majority of coronary artery stenoses will be unrelated to RT; a strong relationship between dose and stenosis would be needed for a positive finding.
It has been proposed that radiation acts in concert, and perhaps in synergy, with other cardiovascular risk factors, such as hypertension, hypercholesterolemia, obesity, and diabetes [Citation20]. A study by Mast et al. showed a higher coronary artery calcium score, an accepted tool to predict coronary artery disease, in a BC cohort investigated pre-RT in comparison to a cohort of healthy women, suggesting women with BC bearing a higher risk of developing coronary artery disease even before RT [Citation21]. In irradiated women with BC, McGale et al. showed a synergy between RT and preexisting IHD, for a new heart disease event to occur [Citation10]. In irradiated as well as non-irradiated patients with coronary artery disease, the majority of stenoses are located in the LAD territory [Citation12]. In view of RT as a cardiovascular risk factor, an increase of the relative risk of stenosis in different parts of the coronary arteries would result in a higher absolute risk of stenosis in LAD, compared to other locations of the coronary arteries.
Our findings are supported by the study by McGale et al. [Citation10]. In a comparison of irradiated left- and right-sided BC, the study showed an increased risk of IHD during the study period of 1977–2001. No differences in IHD incidence was seen in patients receiving RT after 1990 compared to patients irradiated before the 1990s. A possible explanation may be that the coronary artery dose rather than the heart dose is the crucial point to develop IHD, as the coronary doses were estimated to be at the same level during the whole study period, whereas the heart doses were lower after 1990. In contrast, the study by Darby et al. showed a dose-dependent increase of IHD correlating with mean heart dose [Citation11]. However, the Darby study was based on doses estimated from the RT charts, not on doses from 3DCRT treatment plans. According to our results, still a considerable radiation dose is delivered to parts of the coronary arteries when irradiating left-sided BC.
In the study by Moignier et al. in 12 patients who had received mediastinal irradiation for Hodgkin lymphoma, the main finding was a correlation between high radiation dose to coronary artery segments and coronary stenosis [Citation22]. One patient in our study had an occlusion of segment 7, corresponding to mid LAD, approximately eight years after RT, with a mean radiation dose of 50 Gy in the segment. In the same patient, no significant stenoses were detected in other segments of the coronary arteries. This may be a random finding, but might illustrate a late radiation vascular effect.
There is no established safe radiation threshold dose for the coronary arteries. The radiation tolerance dose for the heart is based on studies concerning radiation-induced pericarditis [Citation8] and cardiovascular mortality [Citation9], and in these circumstances the whole heart is the OAR. The Danish Breast Cancer Cooperative Group (DBCG) guidelines [Citation23], based on the study by Gagliardi et al. [Citation9], has suggested the following restriction criteria for heart irradiation in BC: less than 5% of the heart volume should receive 40 Gy (Vheart 40 Gy = 5%) and less than 10% of the heart volume should receive 20 Gy (Vheart 20 Gy = 10%). All the women with right-sided BC, but only 4/7 women with left-sided BC in the study fulfilled these dose constraints. However, the four women with left-sided BC who did fulfill the DBCG heart dose constraints still had high hotspot doses of 46–52 Gy in mdLAD, which we believe is a critical vascular structure for developing late radiation effects. Thus, restriction of doses to the whole heart as an OAR does not exclude high point doses in the coronary arteries, also shown by others [Citation18,Citation24,Citation25].
In conclusion, we found a marked difference of radiation dose distribution in mdLAD between women with left- and right-sided BCs. Tangential RT to the left breast without reg LN irradiation yielded coronary artery maximum doses of approximately 50 Gy to distal LAD, probably not safe concerning late radiation vascular effects. The radiation tolerance dose for the coronary arteries is still uncertain and a large study with long-term follow-up is needed to establish a dose–response relationship between radiation dose and coronary artery stenosis.
Supplementary_Table_S1.docx
Download MS Word (18 KB)Supplementary_Figure_S1.tif
Download TIFF Image (60.5 KB)Acknowledgments
The authors gratefully acknowledge Ann-Louise Jacobsson and Wiviann Björklund for their skilled assistance in collecting medical records and Carina Öberg Kreuger and Per Hållström for retrieval of the CT dose plans and assistance in dose calculations.
Disclosure statement
None of the authors have conflicts of interest regarding the content of this paper.
Funding
This study was supported by grants from the Research Fund of the Department of Oncology, Uppsala University Hospital, the Lions Cancer Foundation, and the Erik, Karin and Gösta Selander Foundation. Lars Holmberg was supported by grants from Swedish Cancer Society and Lars Holmberg and Hans Garmo were supported by Cancer Research UK grants.
ORCID Hans Garmo http://orcid.org/0000-0001-7181-7083
References
- Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011;378:1707–16.
- McGale P, Taylor C, Correa C, Cutter D, Duane F, Ewertz M, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014;383:2127–35.
- Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;366:2087–106.
- Henson KE, McGale P, Taylor C, Darby SC. Radiation-related mortality from heart disease and lung cancer more than 20 years after radiotherapy for breast cancer. Br J Cancer 2013;108:179–82.
- Borger JH, Hooning MJ, Boersma LJ, Snijders-Keilholz A, Aleman BM, Lintzen E, et al. Cardiotoxic effects of tangential breast irradiation in early breast cancer patients: the role of irradiated heart volume. Int J Radiat Oncol Biol Phys 2007;69:1131–8.
- Doyle JJ, Neugut AI, Jacobson JS, Wang J, McBride R, Grann A, et al. Radiation therapy, cardiac risk factors, and cardiac toxicity in early-stage breast cancer patients. Int J Radiat Oncol Biol Phys 2007;68:82–93.
- Stewart JR, Fajardo LF, Gillette SM, Constine LS. Radiation injury to the heart. Int J Radiat Oncol Biol Phys 1995;31:1205–11.
- Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991;21:109–22.
- Gagliardi G, Lax I, Ottolenghi A, Rutqvist LE. Long-term cardiac mortality after radiotherapy of breast cancer-application of the relative seriality model. Br J Radiol 1996;69:839–46.
- McGale P, Darby SC, Hall P, Adolfsson J, Bengtsson NO, Bennet AM, et al. Incidence of heart disease in 35,000 women treated with radiotherapy for breast cancer in Denmark and Sweden. Radiother Oncol 2011;100:167–75.
- Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Bronnum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;368:987–98.
- Nilsson G, Holmberg L, Garmo H, Duvernoy O, Sjogren I, Lagerqvist B, et al. Distribution of coronary artery stenosis after radiation for breast cancer. J Clin Oncol 2012;30:380–6.
- Nilsson G, Holmberg L, Garmo H, Terent A, Blomqvist C. Increased incidence of stroke in women with breast cancer. Eur J Cancer 2005;41:423–9.
- Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, et al. A reporting system on patients evaluated for coronary artery disease. Report of the ad hoc committee for grading of coronary artery disease, council on cardiovascular surgery, American heart association. Circulation 1975 Apr;51:5–40.
- Jansson T, Lindman H, Nygard K, Dahlgren CV, Montelius A, Oberg-Kreuger C, et al. Radiotherapy of breast cancer after breast-conserving surgery: an improved technique using mixed electron-photon beams with a multileaf collimator. Radiother Oncol 1998;46:83–9.
- Feng M, Moran JM, Koelling T, Chughtai A, Chan JL, Freedman L, et al. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys 2011;79:10–8.
- Taylor CW, Nisbet A, McGale P, Goldman U, Darby SC, Hall P, et al. Cardiac doses from Swedish breast cancer radiotherapy since the 1950s. Radiother Oncol 2009;90:127–35.
- Taylor CW, Povall JM, McGale P, Nisbet A, Dodwell D, Smith JT, et al. Cardiac dose from tangential breast cancer radiotherapy in the year 2006. Int J Radiat Oncol Biol Phys 2008;72:501–7.
- Cozzi L, Buffa FM, Fogliata A. Dosimetric features of linac head and phantom scattered radiation outside the clinical photon beam: experimental measurements and comparison with treatment planning system calculations. Radiother Oncol 2001;58:193–200.
- Schultz-Hector S, Trott KR. Radiation-induced cardiovascular diseases: is the epidemiologic evidence compatible with the radiobiologic data? Int J Radiat Oncol Biol Phys 2007;67:10–18.
- Mast ME, Heijenbrok MW, Petoukhova AL, Scholten AN, Schreur JH, Struikmans H. Preradiotherapy calcium scores of the coronary arteries in a cohort of women with early-stage breast cancer: a comparison with a cohort of healthy women. Int J Radiat Oncol Biol Phys 2012;83:853–8.
- Moignier A, Broggio D, Derreumaux S, Beaudre A, Girinsky T, Paul JF, et al. Coronary stenosis risk analysis following Hodgkin lymphoma radiotherapy: a study based on patient specific artery segments dose calculation. Radiother Oncol 2015;117:467–72.
- Thomsen MS, Berg M, Nielsen HM, Pedersen AN, Overgaard M, Ewertz M, et al. Post-mastectomy radiotherapy in Denmark: from 2D to 3D treatment planning guidelines of The Danish breast cancer cooperative group. Acta Oncol 2008;47:654–61.
- Aznar MC, Korreman SS, Pedersen AN, Persson GF, Josipovic M, Specht L. Evaluation of dose to cardiac structures during breast irradiation. Br J Radiol 2011;84:743–6.
- Krueger EA, Schipper MJ, Koelling T, Marsh RB, Butler JB, Pierce LJ. Cardiac chamber and coronary artery doses associated with postmastectomy radiotherapy techniques to the chest wall and regional nodes. Int J Radiat Oncol Biol Phys 2004;60:1195–203.
