Abstract
Background: We conducted a population-based study to establish the incidence, treatment and overall survival over time of patients with small bowel adenocarcinoma.
Material and methods: All patients diagnosed with small bowel adenocarcinoma in the Netherlands between 1999 and 2013 were included (n = 1775). Age-standardized incidence rates were calculated per 100 000 person-years using the European standardized population rate. The influence of patient and tumor characteristics on the administration of chemotherapy was analyzed by means of a multivariable logistic regression analysis. The Cochran-Armitage trend test was conducted to evaluate trends in treatment and survival and the Cox proportional hazards model was used to identify prognostic factors of overall survival.
Results: The incidence of small bowel adenocarcinomas increased, mainly due to an almost twofold increase of duodenal adenocarcinomas. Patients with locoregional duodenal tumors were less likely to undergo surgery (58%), towards 95% of the locoregional jejunal and ileal tumors (p < 0.0001). The use of chemotherapy doubled for adjuvant (7–15%) and palliative chemotherapy (19–37%). Median overall survival of patients with locoregional disease increased from 19 to 34 months (p = 0.0006), whereas median overall survival of patients with metastatic disease remained 4–5 months. Favorable prognostic factors for prolonged survival in locoregional disease, identified by multivariable survival analysis, included age <60 years, tumor stage I or II, diagnosis in 2009–2013, surgical treatment and chemotherapy. Favorable prognostic factors for prolonged survival in metastatic disease were age <50 years, jejunal tumors, surgical treatment and chemotherapy.
Conclusion: Small bowel adenocarcinomas are rare tumors with an increasing incidence. The administration of adjuvant and palliative chemotherapy doubled, but median overall survival only increased for patients with locoregional disease. Given the rarity and dismal prognosis, it is important to develop international studies to determine the optimal treatment for these patients.
Small bowel tumors are rare malignant tumors, accounting for less than 5% of all gastro-intestinal tumors, but the incidence is rising [Citation1]. Small bowel tumors have an unequal distribution in the small bowel. The preferred location depends on the histological subtype. The four major subtypes of small bowel tumors are adenocarcinomas, neuroendocrine tumors (including carcinoids), gastro-intestinal stromal tumors (GIST) and lymphomas [Citation2]. Adenocarcinomas and neuroendocrine tumors are the most common subtypes in the small bowel, both accounting for approximately 40% of small bowel tumors [Citation3–5].
Patients with small bowel adenocarcinomas merely present with non-specific symptoms, such as vague abdominal pain, weight loss, nausea and vomiting, bowel obstruction, gastro-intestinal bleeding or anemia, which challenges the diagnosis. Known predisposing risk factors for these tumors are autoimmune disorders including celiac disease, Crohn’s disease and several hereditary cancer syndromes, including familial adenomatous polyposis (FAP), hereditary nonpolyposis colorectal cancer (HNPCC) and the Peutz-Jeghers syndrome. These predisposing genetic disorders also play a role in the pathogenesis of colon cancer. Although the precise pathogenesis of small bowel adenocarcinomas is unknown, most available data suggest a carcinoma sequence driven multistep process of specific genetic changes similar to colorectal cancers [Citation5–9].
Due to the rarity of the disease, data about small bowel adenocarcinomas are scarce, diverse and contradictory. Therefore, we conducted a population-based study to establish the incidence, treatment and overall survival over time of patients with a small bowel adenocarcinoma in the Netherlands between 1999 and 2013.
Material and methods
Data collection
For this study, data were retrieved from the Netherlands Cancer Registry (NCR), which is managed by the Comprehensive Cancer Organization the Netherlands. The nationwide NCR covers nearly 17 million inhabitants and comprises population-based data on all newly diagnosed malignancies. Primary source of notification of the NCR is the automated nationwide pathological archive (PALGA), supplemented with data from the National Registry of Hospital Discharge Diagnoses. Required information on diagnosis, treatment, patient- and tumor characteristics are routinely extracted from hospital medical records by specially trained registrars operating on behalf of the NCR.
Patients were included if they were diagnosed between 1999 and 2013 with an adenocarcinoma of the small bowel, according to the third version of the International Classification of Disease for Oncology (ICD-O) (topography code C17). Tumors were classified as adenocarcinomas with the following morphology codes: 8140, 8144, 8145, 8210, 8255, 8260, 8261, 8263, 8480, 8481, 8490, 8560, 8570, and 8574. Patients with adenocarcinomas arising from a Meckel’s diverticulum, as well as patients with neuroendocrine tumors, gastro-intestinal stromal tumors, lymphomas or undifferentiated tumors in the small bowel were excluded from analysis.
All adenocarcinomas were classified according to the Tumor Lymph Node Metastasis (TNM) classification and were staged following the recommendations of the International Union Against Cancer in the respective period. The tumors were categorized in two groups, either as locoregional (T1-4N0-2M0) or metastatic cancer (T1-4N0-2M1).
Vital status of patients at 1 January 2014 was assessed through linkage with civil municipal registries and the central bureau for genealogy, which collects data on all deceased Dutch inhabitants. Survival was computed based on all-cause mortality.
Statistical analysis
Descriptive statistics were used to describe the patient and tumor characteristics. Differences in certain tumor characteristics and treatment between the locoregional and metastatic group were compared and analyzed using a two-sided χ2-test. To evaluate trends in treatment and survival, patients were first categorized in three groups by period of diagnosis (1999–2003, 2004–2008 and 2009–2013), and subsequently, trends between the subgroups were analyzed by means of a Cochran-Armitage trend test.
Age-standardized incidence rates were calculated per 100 000 person-years using the European standardized population rate (ESR) for the respective study period. Estimated annual percentage changes (EAPCs) in incidence were estimated by Poisson regression models. The independent influence of relevant patient and tumor characteristics on the administration of chemotherapy for patients with locoregional and metastatic disease was analyzed by means of a multivariable logistic regression analysis, including the 95% confidence interval (CI).
Survival time was defined as the time from date of diagnosis to death. Patients who were lost to follow-up or still alive at 1 January 2014 were censored. Evaluation of significant differences of survival between the subgroups occurred by means of a log-rank test. Multivariable survival analyses, using the Cox proportional hazards model, were carried out to identify independent prognostic factors of overall survival. In order to investigate the effect of therapy on the hazard ratios (HR) of dying, two separate multivariable models were run with and without treatment variables (surgery yes vs. no and chemotherapy yes vs. no). HRs were presented with 95% confidence intervals.
The statistical package SAS Statistical software (version 9.4, SAS institute, Cary, NC, USA) was used to analyze the data. For all statistical tests, a two-sided p-value p < 0.05 was considered statistically significant.
Results
A total of 3930 patients were diagnosed with a small bowel tumor between 1 January 1999 and 31 December 2013. The most common histological subtype was adenocarcinoma, accounting for 1775 cases (45%), followed by neuroendocrine tumors (1429 patients, 36%) and gastro-intestinal stromal tumors (529 patients, 13%). The 1775 patients diagnosed with an adenocarcinoma were enrolled in this study.
The patients’ characteristics are summarized in . We found an equal gender distribution, the median age at time of diagnosis was 69 (range 17–97). The tumors were mainly located in the duodenum (58%), and, respectively, 19% and 14% in the jejunum and the ileum. A comparison between patients diagnosed with tumors located in the duodenum versus patients with tumors located elsewhere in the small bowel showed that patients with tumors in the duodenum were often slightly older and more frequently had a tumor with a higher or unknown tumor stage.
Table 1. Characteristics of patients diagnosed with a small bowel adenocarcinoma, by primary tumor localization (n = 1775).
The age-standardized incidence of small bowel adenocarcinomas increased from 0.5 per 100 000 inhabitants in 1999 to 0.7 per 100 000 inhabitants in 2013 with an estimated annual percentage change (EAPC) of 3.7% (p < 0.001). The increased incidence of small bowel adenocarcinomas is mainly caused by a twofold increase of duodenal adenocarcinomas from 233 in 1999–2003 to 478 cases in 2009–2013 (p = 0.013) ().
Figure 1. Primary tumor location within the small bowel for patients diagnosed with a small bowel adenocarcinoma between 1999 and 2013 in the Netherlands according to period of diagnosis (n = 1775).
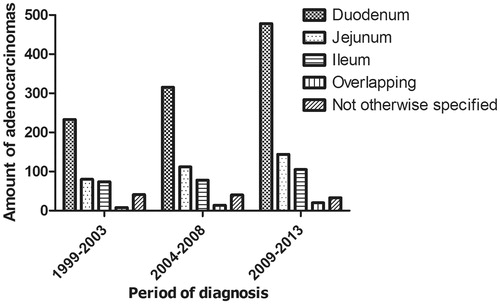
Thirty-three percent of the patients had metastatic disease. Over time the proportion of patients presenting with metastases increased from 27% in 1999–2003 to 38% in 2009–2013 (p < 0.0001). Moreover, the percentage of patients presenting with metastases in multiple organs increased as well from 8% in 1999–2003 to 28% in 2009–2013 (p = 0.0003). The most common metastatic site was the liver (46%), followed by the peritoneal cavity (29%) and extra regional lymph nodes (12%). Patients with metastatic disease arising from duodenal origin showed a different metastatic pattern compared to patients with primary tumors located elsewhere in the small bowel. The majority of patients with metastatic duodenal adenocarcinomas had metastases located in the liver (54%), whereas in patients with metastases from non-duodenal adenocarcinomas the peritoneal cavity was the most frequently affected site (44%).
In the group of patients with locoregional disease, 73% underwent a surgical resection of the primary tumor in contrast to 30% of the patients with metastatic disease (p < 0.0001). The percentage of patients with locoregional disease undergoing a resection slightly increased from 71% in 1999–2003 to 77% in 2009–2013, while the percentage of patients with metastatic disease undergoing a surgical resection of the primary tumor decreased from 38% to 25% (p = 0.0031).
Tumor location was an important predictive factor for surgery. In locoregional disease, 58% of the patients with duodenal carcinomas underwent a surgical intervention with curative intent as compared to 95% of the patients with jejunal and ileal carcinomas (p < 0.0001). The percentage of patients with duodenal adenocarcinomas undergoing surgery increased from 54% to 64% throughout the study period (p = 0.0179). In metastatic disease, only 7% of the patients with duodenal adenocarcinomas underwent surgery, in contrast to respectively 63% and 81% of the patients with jejunal and ileal tumors (p < 0.0001). Other palliative interventions, such as a bilio-digestive or intestinal bypass, endoscopic stent placement or celiac plexus block, were performed in 24% of the patients with metastatic duodenal adenocarcinomas, and respectively in 5% and 6% of the patients with metastatic jejunal and ileal tumors. In addition, 14% of the patients with locoregional duodenal adenocarcinomas received a palliative intervention.
Eleven percent of the patients with locoregional disease received chemotherapy, while 33% of the patients with metastatic disease did. The use of chemotherapy increased over time for patients with locoregional disease from 7% in 1999–2003 to 15% in 2009–2013 (p = 0.0001). Of the 91 patients with locoregional disease, undergoing both surgical resection and chemotherapy, the majority received the chemotherapy in the adjuvant setting. Multivariable logistic regression analyzes showed that chemotherapy in patients with locoregional disease was more often offered to younger patients, patients with ileal or stage III tumors or patients who were diagnosed in the period 2009–2013 (). In patients with metastatic disease the prescription of palliative chemotherapy significantly increased from 19% in 1999–2003 to 37% in 2009–2013 (p = 0.001). In metastatic disease, younger patients and patients who were diagnosed after 2003 received chemotherapy more frequently ().
Table 2. Crude percentages and adjusted odds for receiving chemotherapy among patients diagnosed with small bowel adenocarcinomas between 1999–2013 in the Netherlands, by extent of disease (n = 1775).
The median overall survival of patients diagnosed with a small bowel adenocarcinoma remained stable around 13–14 months, with one and five year survival rates of 53% and 25% respectively. Patients with locoregional disease had a median overall survival of 25 months (one and five year survival rates 65% and 36% respectively). The median overall survival of patients with locoregional disease increased from 19 months in the first period to 34 months in the last period (p = 0.0006). In patients with locoregional disease who underwent a surgical resection, a median overall survival of 48 months was observed. Whereas patients receiving (neo-)adjuvant chemotherapy in combination with surgery exhibited a significantly better median overall survival of 66 months (p = 0.0338).
The median overall survival of patients with metastatic disease remained stable around 4–5 months (one and five year survival rates 26% and 3% respectively). A median overall survival of 10 months was seen in patients with metastatic disease who were treated with palliative chemotherapy, in contrast to a 3 month median overall survival in patients who did not receive palliative chemotherapy.
Favorable prognostic factors, identified by a separate multivariable survival analysis, including patients with locoregional disease, were age <60 years, low tumor stage (stage I, II) and diagnosis in the period 2009–2013 (). Factors that were associated with poor survival included age ≥70 years, tumor localization in the duodenum and an unknown tumor stage (stage X). Surgical treatment and chemotherapy, were added separately to the model to investigate its effect on the hazard ratio of death according to period of diagnosis and different patient and tumor characteristics. Surgical treatment and chemotherapy were both favorable prognostic factors. Remarkably, after adjustment for surgery only, a tumor located in the duodenum was no longer a negative prognostic factor and diagnosis in the period 2004–2008 became a positive prognostic factor. Chemotherapy did not influence the effect of the other characteristics on the hazard ratio of death.
Table 3. Crude median overall survival, crude 1-year survival, crude 5-year survival, adjusted hazard ratios with and without adjustment for treatment for patients diagnosed with locoregional small bowel adenocarcinoma between 1999 and 2013 in the Netherlands (n = 1194).
In a multivariable survival analysis without adjustment for treatment including patients with metastatic disease, age <50 years and primary tumor located in the jejunum or ileum were positive prognostic factors (). Age >80 years was the only negative prognostic factor. No beneficial influence of time was seen. After adjustment for chemotherapy and surgery, both positive prognostic factors, a primary tumor located in the ileum became a negative prognostic factor.
Table 4. Crude median overall survival, crude 1-year survival, crude 5-year survival, adjusted hazard ratios with and without adjustment for treatment for patients diagnosed with metastatic small bowel adenocarcinoma between 1999 and 2013 in the Netherlands (n = 581).
Discussion
This population-based study examined the incidence, treatment and median overall survival over time in patients diagnosed with a small bowel adenocarcinoma in the Netherlands between 1999 and 2013 and is one of the largest conducted studies in the field of small bowel adenocarcinomas so far. Our study showed that the incidence of small bowel adenocarcinomas is rising. Furthermore, we found that the resection rates in non-metastatic small bowel cancer increased and the median overall survival in patients with locoregional disease improved over time. The median overall survival of patients with metastatic disease remained stable, despite the increased treatment with palliative chemotherapy.
The distribution pattern of small bowel adenocarcinomas throughout the intestine was comparable with previous studies [Citation3–5,Citation7]. It has been hypothesized that the duodenum might be more susceptible for carcinogenesis than the jejunum and ileum due to the metabolism or dilution of ingested carcinogens in transit through the small bowel or interactions of the carcinogens with the pancreaticobiliary secretions [Citation3,Citation7,Citation10,Citation11].
Based on our comparison between patients diagnosed with tumors located in the duodenum versus patients diagnosed with tumors located elsewhere in the small bowel, it could be questioned whether these tumors should be considered as one entity. Patients with tumors located in the duodenum are often slightly older, have more advanced disease stages and have a different metastatic pattern.
A slight increase in the incidence of small bowel adenocarcinomas was seen between 1999 and 2013, which is mainly caused by the twofold increase of duodenal adenocarcinomas. The exact cause for the specific increase in duodenal adenocarcinomas is unknown. Partially it can be explained by improved diagnostics, resulting in a reduction of misclassification of duodenal adenocarcinomas as pancreatic tumors and adenocarcinoma of unknown primary (ACUP) [Citation10,Citation12]. The modified food consumption might have attributed to increased incidence rates as well. Previous studies found sugar, refined carbohydrates, red meat and smoked food to be associated with the development of small bowel adenocarcinomas [Citation2,Citation11].
The percentage of patients diagnosed with metastatic disease increased over time, which can be explained by stage migration caused by new and improved diagnostics, such as multidetector row computed tomography scans (MDCT) and magnetic resonance (MR) enteroclysis [Citation13].
Surgical resection is the only therapy for potential cure in small bowel adenocarcinoma [Citation2]. In line with previous studies, 73% of the patients with locoregional disease underwent an intentionally curative resection [Citation5,Citation7]. Resection rates were higher in jejunal and ileal tumors compared to resection rates in duodenal tumors, since surgical resection of upper duodenal tumors requires a pancreaticoduodenectomy, which is specialized major surgery in comparison to the more simple segmental resections with removal of surrounding tissue for jejunal and ileal tumors [Citation5,Citation7].
Over time the resection rates increased, especially due to an increased number of resections in patients with duodenal tumors. We hypothesize that may be due to the centralization of pancreaticoduodenectomies in The Netherlands [Citation14,Citation15]. The amount of surgical interventions in patients with metastatic disease decreased drastically, which is probably the result of improved palliative interventions, such as endoscopically placed (bilio-)duodenal endoprotheses, and the increased use of chemotherapy [Citation16]. Palliative interventions in patients with non-metastatic small bowel cancer were mostly performed in patients with duodenal adenocarcinomas, which are more often irresectable compared to jejunal and ileal tumors [Citation7].
The proportion of patients receiving chemotherapy doubled during the study period, both for patients with locoregional and metastatic disease. Especially in patients with locoregional disease the twofold increase is remarkable, since non-observational studies addressing the beneficial effect of chemotherapy are lacking. Overman et al found adjuvant chemotherapy to be associated with an improvement of disease free survival, but not with improvement of overall survival [Citation17]. Recently, a population-based study conducted by Ecker et al showed a survival benefit of 16 months (42 vs 26 months) for patients with stage III tumors treated with adjuvant chemotherapy [Citation18]. We demonstrate that in patients with locoregional disease chemotherapy was more often offered to younger patients, patients with ileal or stage III tumors and patients who were diagnosed in the period 2009–2013. In metastatic disease however, the doubling of palliative chemotherapy is not surprising, since a survival benefit of several months has already been observed in multiple retrospective studies [Citation5,Citation19–21]. In patients with metastatic disease, only a younger age and diagnosis after 2003 were positive predictive factors for receiving palliative chemotherapy.
The overall survival rate of all patients with an adenocarcinoma of the small bowel did not improve over time and remained dismal with an overall median survival of 13–14 months. Our results are inferior to the reported overall survival of approximately 20 months in other population-based studies, but these studies were merely conducted before the millennium and might have included neuroendocrine tumors with a more indolent behavior [Citation5,Citation7,Citation22,Citation23].
The median overall survival of patients with locoregional disease improved from 19 months in 1999–2003 to 34 months in 2009–2013, which might be explained by stage migration, increased use of chemotherapy and the centralization of pancreatic cancer surgery. Moreover, we found that patients treated with adjuvant chemotherapy after surgical resection had significant higher survival rates, 66 months compared to 48 months for patients not treated with adjuvant chemotherapy. However, it should be noted that the amount of patients receiving both treatments were limited in our study. Furthermore, selection bias might have played a role as well. Fitter patients, those with a better survival beforehand, might have received chemotherapy more frequently. Other favorable prognostic factors for prolonged survival in patients with locoregional disease, identified by multivariable analysis, were age <60 years, tumor stage I and II, surgical treatment and chemotherapy. These findings are comparable to previously determined prognostic factors [Citation3,Citation5,Citation7,Citation20,Citation22,Citation24]. In addition, in patients with locoregional disease, duodenal tumors appeared to be an adverse prognostic factor in multivariable analysis without adjustment for treatment. However, after adjustment for surgery only, a duodenal tumor was not a negative prognostic factor anymore, which implies that the poor prognosis of these tumors is the result of the relative lack of possibilities for surgical intervention.
In metastatic disease the median overall survival remained stable around 4–5 months despite doubling of the prescription of palliative chemotherapy from 19% to 37% in the recent years. In patients with metastatic disease, favorable prognostic factors identified by multivariable analysis included age <50 years, primary tumor located in the jejunum, surgical treatment and chemotherapy. These prognostic factors are also consistent to previously published data [Citation3,Citation7,Citation22].
A limitation of our study is that detailed information on performance status, nutritional status, disease related symptoms, the specific tumor localization within the duodenum, type of chemotherapy and type of surgical and palliative intervention are lacking, due to the population based nature of our data. However, our results did not differ from other studies [Citation3–5,Citation7].
In conclusion, small bowel adenocarcinomas are rare tumors with an increasing incidence, mainly caused by the rise of duodenal adenocarcinomas. The median overall survival of patients with locoregional disease improved significantly over time, which might be due to the increasing use of chemotherapy and the implementation of centralizing pancreatic cancer surgery. However, the median overall survival of patients with metastatic disease remained stable, despite doubling the administration of palliative chemotherapy. Due to the rarity and dismal prognosis of this disease, it is of importance to develop international studies to determine the optimal treatment for these patients. The differences found in characteristics and median overall survival between patients diagnosed with tumors located in the duodenum and tumors located elsewhere in the small bowel might suggest that in future research both should be considered as different entities.
Acknowledgements
The authors thank the registration team of the Netherlands Cancer Registry for their dedicated data collection.
Disclosure statement
None to declare.
References
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin 2008;58:71–96.
- Aparicio T, Zaanan A, Svrcek M, Laurent-Puig P, Carrere N, Manfredi S, et al. Small bowel adenocarcinoma: epidemiology, risk factors, diagnosis and treatment. Dig Liver Dis 2014;46:97–104.
- Bilimoria KY, Bentrem DJ, Wayne JD, Ko CY, Bennett CL, Talamonti MS. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg 2009;249:63–71.
- Chow JS, Chen CC, Ahsan H, Neugut AI. A population-based study of the incidence of malignant small bowel tumours: SEER, 1973-1990. Int J Epidemiol 1996;25:722–8.
- Dabaja BS, Suki D, Pro B, Bonnen M, Ajani J. Adenocarcinoma of the small bowel: presentation, prognostic factors, and outcome of 217 patients. Cancer 2004;101:518–26.
- Chang HK, Yu E, Kim J, Bae YK, Jang KT, Jung ES, et al. Adenocarcinoma of the small intestine: a multi-institutional study of 197 surgically resected cases. Hum Pathol 2010;41:1087–96.
- Howe JR, Karnell LH, Menck HR, Scott-Conner C, The American College of Surgeons Commission on Cancer and the American Cancer Society. Adenocarcinoma of the small bowel: review of the National cancer data base, 1985-1995. Cancer 1999;86:2693–706.
- Neely D, Ong J, Patterson J, Kirkpatrick D, Skelly R. Small intestinal adenocarcinoma: rarely considered, often missed? Postgrad Med J 2013;89:197–201.
- Shenoy S. Primary small-bowel malignancy: update in tumor biology, markers, and management strategies. J Gastrointest Cancer 2014;45:421–30.
- Lu Y, Frobom R, Lagergren J. Incidence patterns of small bowel cancer in a population-based study in Sweden: increase in duodenal adenocarcinoma. Cancer Epidemiol 2012;36:e158–63.
- Negri E, Bosetti C, La Vecchia C, Fioretti F, Conti E, Franceschi S. Risk factors for adenocarcinoma of the small intestine. Int J Cancer 1999;82:171–4.
- Mnatsakanyan E, Tung WC, Caine B, Smith-Gagen J. Cancer of unknown primary: time trends in incidence, United States. Cancer Causes Control 2014;25:747–57.
- Anzidei M, Napoli A, Zini C, Kirchin MA, Catalano C, Passariello R. Malignant tumours of the small intestine: a review of histopathology, multidetector CT and MRI aspects. Br J Radiol 2011;84:677–90.
- Lemmens VE, Bosscha K, van der Schelling G, Brenninkmeijer S, Coebergh JW, de Hingh IH. Improving outcome for patients with pancreatic cancer through centralization. Br J Surg 2011;98:1455–62.
- Nienhuijs SW, van den Akker SA, de Vries E, de Hingh IH, Visser O, Lemmens VE. Nationwide improvement of only short-term survival after resection for pancreatic cancer in the Netherlands. Pancreas 2012;41:1063–6.
- Kaw M, Singh S, Gagneja H. Clinical outcome of simultaneous self-expandable metal stents for palliation of malignant biliary and duodenal obstruction. Surg Endosc 2003;17:457–61.
- Overman MJ, Kopetz S, Lin E, Abbruzzese JL, Wolff RA. Is there a role for adjuvant therapy in resected adenocarcinoma of the small intestine. Acta Oncol 2010;49:474–9.
- Ecker BL, McMillan MT, Datta J, Mamtani R, Giantonio BJ, Dempsey DT, et al. Efficacy of adjuvant chemotherapy for small bowel adenocarcinoma: a propensity score-matched analysis. Cancer 2016;122:693–701.
- Fishman PN, Pond GR, Moore MJ, Oza A, Burkes RL, Siu LL, et al. Natural history and chemotherapy effectiveness for advanced adenocarcinoma of the small bowel: a retrospective review of 113 cases. Am J Clin Oncol 2006;29:225–31.
- Khan K, Peckitt C, Sclafani F, Watkins D, Rao S, Starling N, et al. Prognostic factors and treatment outcomes in patients with small bowel adenocarcinoma (SBA): The Royal Marsden Hospital (RMH) experience. BMC Cancer 2015;15:15.
- Koo DH, Yun SC, Hong YS, Ryu MH, Lee JL, Chang HM, et al. Systemic chemotherapy for treatment of advanced small bowel adenocarcinoma with prognostic factor analysis: retrospective study. BMC Cancer 2011;11:205.
- Nicholl MB, Ahuja V, Conway WC, Vu VD, Sim MS, Singh G. Small bowel adenocarcinoma: understaged and undertreated? Ann Surg Oncol 2010;17:2728–32.
- Scherubl H, Jensen RT, Cadiot G, Stolzel U, Kloppel G. Neuroendocrine tumors of the small bowels are on the rise: early aspects and management. World J Gastrointest Endosc 2010;2:325–34.
- Wu TJ, Yeh CN, Chao TC, Jan YY, Chen MF. Prognostic factors of primary small bowel adenocarcinoma: univariate and multivariate analysis. World J Surg 2006;30:391–8.
