Abstract
Background: Oxaliplatin-induced peripheral neuropathy (OIPN) of acute and chronic type is well known, but long-term chronic type OIPN and its impact on quality of life (QoL) has not been extensively studied. Clinical experience indicates that oxaliplatin tolerance might vary with climate.
Material and methods: Patient-reported chronic type OIPN and QoL among patients treated with oxaliplatin added to a fluoropyrimidine (Folfox or Capox) in the adjuvant setting of colorectal cancer (CRC) were assessed in a single center cross-sectional study by using the EORTC QLQ-CIPN20 and QLQ-C30 questionnaires. Comparison was made to patients treated with a fluoropyrimidine (5-FU or capecitabine) alone during the same time period.
Results: Of 161 patients being disease-free 1–8 years after stop of treatment and invited, 84% participated; 65 treated with oxaliplatin and 71 with a fluoropyrimidine alone. Mean cumulative oxaliplatin dose was 567 mg/m2 (55% of planned dose). Oxaliplatin-treated patients reported statistically and clinically significant worse sensory as well as motor scale scores, dominated by symptoms from the feet. Severe tingling and numbness in toes/feet was reported by 38% and 37%, respectively, by oxaliplatin-treated patients compared with 8% for both by fluoropyrimidine alone patients (p < 0.001). Subgroup analyses indicated no impact of gender, age, regimen, time since stop of treatment or cumulated oxaliplatin dose for severity of the chronic type OIPN. The oxaliplatin compared with the fluoropyrimidine group reported worse QoL scores throughout all domains, with statistically and clinically significant differences for role and social function, nausea/loss of appetite and financial problems.
Conclusions: Oxaliplatin added to a fluoropyrimidine for adjuvant treatment of CRC in a country with subarctic climate is associated with long-term, seemingly chronic, sensory neuropathy and impairment of QoL. This should be taken into account in clinical decision making on oxaliplatin treatment in the adjuvant setting.
Adjuvant treatment to eradicate microscopic disease is now standard following radical resection of colorectal cancer (CRC) with prognostic features indicating relevant risk of relapse and with the aim to improve disease-free and overall survival. However, the indications for adjuvant treatment in CRC might be about to change towards less use in light of the lower risk for recurrence now observed due to improved surgery and stage migration [Citation1,Citation2]. The adjuvant treatment was previously 5-fluorouracil (5-FU) alone but is now often combined with oxaliplatin as its addition improves long-term disease control [Citation3–6]. However, this benefit comes at the price of the now well established adverse effects of acute and chronic oxaliplatin-induced peripheral neuropathy (OIPN) [Citation7,Citation8].
The acute OIPN, characterized by cold-induced tingling in hands and feet, sometimes also combined with oropharyngeal dysesthesia, is troublesome yet reversible and therefore mostly of less clinical magnitude. The chronic OIPN with sensory impairment, tingling and pain, however, is potentially more problematic in adjuvant patients with long life expectancy.
The chronic OIPN was in the initial pivotal trials on the addition of oxaliplatin to 5-FU or capecitabine described to be modest with respect to intensity and duration, with most patients recovering within 1–2 years from stop of treatment [Citation4,Citation9,Citation10]. However, more recent retrospective follow-up studies indicate more severe problems with chronic type OIPN, both with respect to grade of symptoms and their duration [Citation11–13].
Whereas most of these follow-up studies focused on assessment of OIPN alone, only few studies have investigated the impact of OIPN on quality of life (QoL) or made comparisons with CRC patients treated in the adjuvant setting with fluoropyrimidine alone. Furthermore, the clinical impression from Sweden with cold winters is that OIPN, both the acute and the chronic type, gets worse in cold weather with the potential implication that the problem of OIPN characterized in one geographical region might not necessarily apply to a region with another type of climate.
Thus, the use of oxaliplatin in the adjuvant treatment of CRC might need to be adjusted to the geographical region in which it is used. However, we are not aware of any study that has investigated the problem of chronic type OIPN when the drug has been used in a country with subarctic climate.
With this background, we considered it relevant to investigate the problem of chronic type OIPN in adjuvant treatment of CRC in a country with cold winters, but also to simultaneously assess the impact of OIPN on QoL and compare the findings with those in patients having the same type of treatment during the same time period and the same weather conditions but with fluoropyrimidine alone.
Material and methods
Setting, participants and patient-reported outcome measures
This was a single center cross-sectional study based on patient-reported outcome measures from established questionnaires. We identified all patients having finished adjuvant treatment for CRC with fluoropyrimidine alone or combined with oxaliplatin during the time period 2004–2011 at Department of Oncology, University Hospital, Uppsala, Sweden. The study period was chosen as oxaliplatin was started to be used in the adjuvant treatment of CRC at our center in 2003 and the period allowed for long-term follow-up of chronic type OIPN. Patients found to be disease-free and alive were invited to participate by submission of an introductory letter explaining the aims of the study and the patient accepted to participate by answering the questionnaires appended. A reminder was sent one month later in the case the patient had not responded. Details on basic demographics, comorbidity, CRC stage and adjuvant treatment were retrieved from the patient files. As a normal reference for symptoms of neuropathy, the questionnaire was also distributed to and answered by 52 seemingly healthy persons (30 males/22 females; mean age 52 years).
Neuropathy was assessed by the EORTC QLQ-CIPN20 questionnaire [Citation14,Citation15] and QoL by the EORTC QLQ-C30 (version 3.0) questionnaire [Citation16]. The former includes 20 questions divided into scales for sensory, motor and autonomic function. For details on questionnaire contents, see Results section. Comorbidity at start of treatment was scored according to the Charlson comorbidity index [Citation17]. The study was approved by the Ethical Committee in Uppsala (Dnr 2012/078).
Treatments
Selection of treatment, such as type of fluoropyrimidine and oxaliplatin or not, for the individual patient was based on general and local guidelines taking into consideration estimated risk for recurrence, patient age, performance status and comorbidity as well as patient preferences regarding adverse effects, treatment schedules and convenience. Patients treated with fluoropyrimidine alone were scheduled for six months of Nordic FLv or capecitabine. Nordic FLv was 5-FU 500 mg/m2 and 100 mg calciumfolinate iv bolus Days 1 and 2 q 2 weeks. Capecitabine was 1250 mg/m2 (1000 mg/m2 for patients >65 years) × 2 for 14 days q 3 weeks. Patients treated with oxaliplatin combined with fluoropyrimidine were scheduled for six months of Nordic Flox, Folfox or Capox. Nordic Flox was Nordic FLv as above with oxaliplatin 85 mg/m2 1 h infusion added Day 1. Folfox was 5-FU 400 mg/m2 iv bolus, oxaliplatin 85 mg/m2 1 h iv infusion and calciumfolinate 200 mg/m2 1 h iv infusion Day 1 with 46 h iv infusion of 2800 mg/m2 5-FU starting Day 1. Capox was capecitabine 1000 mg/m2 × 2 for 14 days q 3 weeks with oxaliplatin 130 mg/m2 1 h iv infusion Day 1. Dose reductions were according to standard clinical principles. Specifically, oxaliplatin was to be reduced and then stopped in the case of symptoms of acute OIPN still remaining at the time for the next oxaliplatin infusion. If oxaliplatin was stopped, the fluoropyrimidine was to be continued for the remains of the six-month treatment period.
Data management and statistics
All 20 items in the CIPN20 questionnaire and the 28 first items in the QLQ-C30 questionnaire are answered with one of the four response categories ‘Not at all’, ‘A little’, ‘Quite a bit’ and ‘Very much’. The QLQ-C30 questions 29 and 30 on global QoL, however, are answered on a seven response category scale. For presentation and statistical calculations, the raw scores were linearly transformed to scores ranging from 0 to 100 in accordance with EORTC guidelines [Citation18]. High scores for the symptom scales and symptom items in the CIPN20 questionnaire as well as for the symptom items in the QLQ-C30 questionnaire represent a high level of symptoms/problems whereas high scores for functional scales and for global QoL in QLQ-C30 represent high/healthy level of functioning or high global QoL. Individual questionnaire items were collected into function or symptom domains in accordance with guidelines [Citation15,Citation18]. The individual items ‘Difficulties using car pedals’ and ‘Difficulties getting or keeping erection’ were left out of the calculations of motor and autonomic function scales, but are presented separately as many patients did not report on those items.
Data are presented as mean values with standard deviations (SD). Statistical comparisons between groups treated with or without oxaliplatin were made with the non-parametric Mann-Whitney test for unpaired data and for the distribution in the treatment groups of patients with severe symptoms, defined as the response ‘Quite a bit’ or ‘Very much’, by Fischer’s exact test. A p-value <0.05 was considered statistically significant and no adjustment was made for multiple analyses. Differences between groups in scale/symptom scores were considered clinically relevant if the ratio score difference observed/SD was approximately 0.5 or higher [Citation19]. For oxaliplatin-treated patients subgroup analyses were made based on gender, age (< or ≥65 years), type of regiment (Flox/Folfox vs. Capox), time (years) since stop of treatment and total cumulated dose of oxaliplatin (mg, quartiles). All statistical data calculation and graphical data presentation were made in GraphPad Prism, version 6.
Results
Demographic and clinical characteristics
Seventy-six patients invited to the study were treated with an oxaliplatin combination and 85 were treated with fluoropyrimidine alone. Sixty-five and 71 patients accepted to participate by return of the questionnaires and the response rate was thus 86% and 84% for the oxaliplatin and fluoropyrimidine groups, respectively. Basic demographic and clinical data for the patients participating are presented in . The patients were evenly distributed with respect to gender whereas, as expected, the oxaliplatin group was clearly younger than the fluoropyrimidine alone group, mean 64 versus 71 years. Comorbidity according to the Charlson index was slightly, but not statistically significantly, higher for the fluoropyrimidine alone group, 0.9 versus 0.8. As expected the oxaliplatin group had more advanced tumors. Fifty-two percent of the oxaliplatin patients were treated with Capox and 82% of fluoropyrimidine alone patients were treated with capecitabine, The mean cumulated dose of oxaliplatin was 567 mg/m2 and only 12% of patients scheduled for treatment with oxaliplatin completed their treatment according to plan. Among the fluoropyrimidine alone patients, however, the majority (69%), completed treatment as planned. The number of patients per time period since stop of treatment was reasonably evenly distributed.
Table 1. Demographics and clinical characteristics of patients included.
Treatment-related neuropathy
We first analyzed the QLQ-CIPN20 questionnaire items according to the established symptom scales (). The oxaliplatin compared with the fluoropyrimidine group reported highly statistically significantly more symptoms related to sensory as well as motor functions. In line with these results oxaliplatin patients also reported more difficulties using car pedals. These differences observed could also be considered clinically relevant. The autonomic scale and erection problems, however, did not differ between the groups, indicating that oxaliplatin does not clearly impair the central nervous system or autonomic nerve function. Sensory, motor as well as autonomic scale scores were statistically significantly (p = 0.0037–<0.0001) lower, and thus better, for the healthy control compared with the fluoropyrimidine alone group. The considerably higher age of patients in the latter might explain this difference [Citation20], although it cannot be excluded that fluoropyrimidines also add to neuropathy.
Table 2. Nerve function according to the EORTC CIPN20 questionnaire, mean (SD).
Based on the differences observed between the oxaliplatin and fluoropyrimidine alone groups in both the sensory and motor scales we analyzed differences in scores for each individual item included in the scores. The oxaliplatin group reported more symptoms for all nine items included in the sensory score with statistical significance for tingling in fingers/hands and toes/feet, numbness and pain in toes/feet, impaired standing/walking and difficulty distinguishing hot from cold (). All but the latter could be considered clinically relevant with the major problems located to the toes/feet as judged from the score levels and differences between the patient groups.
Figure 1. Symptom scores for sensory (A) and motor (B) items in the EORTC QLQ-CIPN20 questionnaire for patients treated with oxaliplatin (black bars) or fluoropyrimidine alone (gray bars). Higher scores mean more symptoms/problems. Data are presented as mean values with standard deviation. ***p < 0.0001; **p < 0.001; *p < 0.05 for comparisons oxaliplatin versus fluoropyrimidine alone groups.
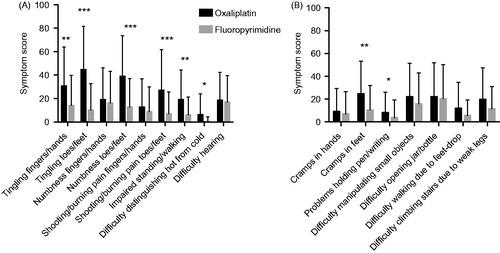
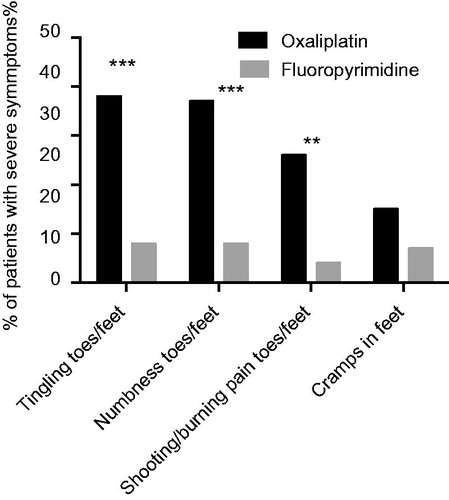
For the motor score items the symptom scores were generally lower, but also for these items the oxaliplatin group reported worse symptoms than the fluoropyrimidine alone group. The differences were mostly small, however, and both statistically and clinically significant only for cramps in feet (). Based on the differences observed for the sensory and motor single items we selected four items considered to best reflect chronic type OIPN for further analysis; tingling in toes/feet, numbness in toes/feet, pain in toes/feet and cramps in feet. Limiting the between group analysis for these items to the fraction of patients in each group reporting more severe symptoms, i.e. ‘Quite a bit’ or ‘Very much’, we observed high scores and statistically and clinically relevant differences for the three sensory items (p < 0.0001, <0.0001 and p = 0.0004, respectively), but not for the motor item (p = 0.1709; ). Combinations of severe sensory symptoms from the feet were frequent in the oxaliplatin group. Thus, 76%, 52% and 48% of patients with severe tingling also had severe numbness, pain or numbness and pain, respectively.
Figure 2. Percent of patients treated with oxaliplatin (black bars) or fluoropyrimidine alone (gray bars) considered to have severe symptoms/problems, i.e. responding ‘Quite a bit’ or ‘Very much’ to the EORTC QLQ-CIPN20 sensory/motor items indicated. ***p < 0.0001; **p < 0.001 for comparisons oxaliplatin versus fluoropyrimidine alone groups.
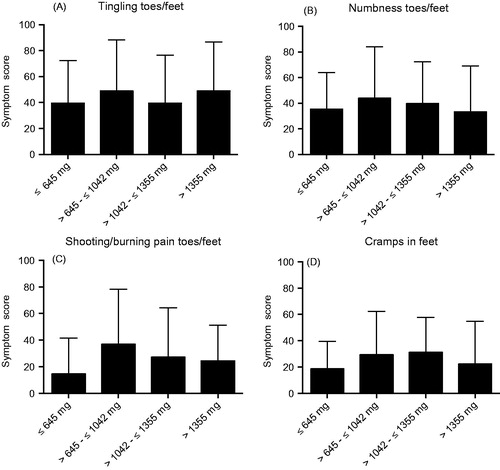
The four items were then used as sensitive markers in the subgroup analyses. There were no obvious differences in scores for these items when oxaliplatin-treated patients were divided in two groups based on gender, age <65 or ≥65 years or type of oxaliplatin-containing regimen (). Mean cumulated oxaliplatin dose was similar for women compared with men (562 vs. 572 mg/m2) and for Flox/Folfox compared with Capox (588 vs. 548 mg/m2). There was also no clear trend for decreasing symptoms or differences between the oxaliplatin and fluoropyrimidine alone groups by time since stop of treatment, although the difference between the groups was smallest after 6–8 years (). Furthermore, there was also no clear trend in these scores to increase by cumulated dose of oxaliplatin ().
Figure 3. Symptom scores for the EORTC QLQ-CIPN20 sensory/motor items indicated for patients treated with oxaliplatin and divided for gender (A), age (B) and type of regimen (C). Higher scores mean more symptoms/problems. Data are presented as mean values with standard deviation.
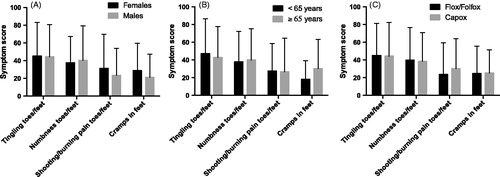
Figure 4. Symptom scores for the EORTC QLQ-CIPN20 items tingling (A), numbness (B), pain (C) and cramps (D) in toes/feet for patients treated with oxaliplatin (black bars) or fluoropyrimidine alone (gray bars) divided into five groups based on time since stop of treatment. Higher score means more symptoms/problems. Data are presented as mean values with standard deviation.
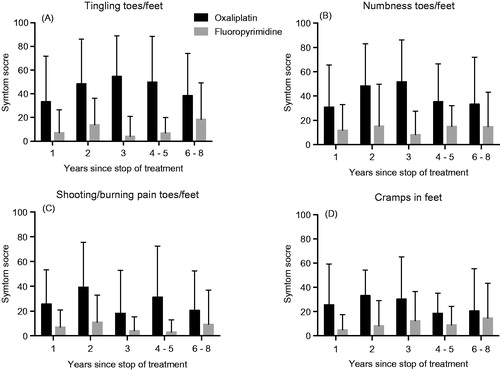
Figure 5. Symptom scores for the EORTC QLQ-CIPN20 items tingling (A), numbness (B), pain (C) and cramps (D) in toes/feet for patients treated with oxaliplatin divided into quartiles for total cumulated dose oxaliplatin received. Higher score means more symptoms/problems. Data are presented as mean values with standard deviation.
Associated effects on quality of life
Somewhat unexpected the oxaliplatin compared with the fluoropyrimidine alone group reported worse QoL for almost every function scale and symptom score included in the QLQ-C30 questionnaire (). The differences were statistically significant for role function, social function, nausea/vomiting, pain and financial problems. Applying the rule of thumb based on score differences and SDs for judgement of clinical relevance [Citation19], all the statistically significant differences in QoL observed could be considered clinically relevant.
Table 3. Health-related quality of life scores according to the EORTC QLQ-C30, mean (SD).
Discussion
Adjuvant chemotherapy in CRC is a delicate balance between benefit in terms of disease-free and overall survival and short- and long-term adverse effects. For fluoropyrimidines alone this balance is mostly in favor of treatment in patients with a reasonably high risk of recurrence in light of the established clinically relevant benefit and low risk of long-term or chronic sequelae from this drug [Citation3]. Oxaliplatin, however, is more troublesome to judge from this balance point of view as the benefit from adding it to a fluoropyrimidine in the adjuvant setting is small [Citation5,Citation6,Citation21] and there is clearly a risk, though not very clearly defined, for long-term impairment of peripheral nerve function without any established effective treatment [Citation7,Citation8]. Thus, it is important to define the risk for long-term OIPN to make it possible to better inform the patients on the benefit/risk balance when making decisions about adjuvant treatment.
The initial reports from the pivotal clinical trials that established benefit of oxaliplatin added to fluoropyrimidine in the adjuvant setting indicated modest and mostly reversible chronic type OIPN. Thus, in the MOSAIC trial OIPN clearly improved by time and at 18 months follow-up mild or moderate sensory loss and paresthesia was reported in 3.4% of the patients and only 0.5% reported neuropathy that interfered with function [Citation4]. In the NSABP C-07 trial OIPN was also clearly reversible with a median time to resolution of nine months and at 18 months follow-up approximately 10% of the patients reported ‘Quite a bit’ or more severe symptoms from the feet [Citation9]. In NO16968 persistent OIPN was vaguely reported in 5% of the patients with a median time to recovery of one month [Citation6]. Furthermore, a systematic review of 14 studies on chronic type OIPN indicated varying but mostly low frequent and mild symptoms [Citation10].
However, retrospective and/or long-term follow-up studies point to more severe chronic type OIPN. Thus, impaired function was reported in 11% of patients at the 12-month follow-up and a Dutch registry data indicated clinically significant neurosensory symptoms mainly from the feet in close to 20–30% of the patients after a mean follow-up of four years [Citation11,Citation12]. Furthermore, at a median follow-up of seven years in the NSABP C-07 trial approximately 15% of oxaliplatin-treated patients reported clinically significant sensory neuropathy in hands and feet [Citation13].
Although not uniform, most studies on chronic type OIPN report worse symptoms by cumulated dose of oxaliplatin and when it comes to type of regimen Folfox has been found worse or equal to Capox [Citation7,Citation10,Citation12,Citation22,Citation23]. Furthermore, the cumulated oxaliplatin dose in the adjuvant trials reporting OIPN has typically been in the range 700–800 mg/m2, corresponding to 70–80% of the dose planned [Citation4–6,Citation10,Citation22,Citation23]. Finally, studies including a comparison between adjuvant fluoropyrimidine alone and with oxaliplatin added to it reported some statistically significant, but mostly rather modest differences in chronic neuropathy severity between these regimens [Citation11,Citation13].
The results from our study on chronic type OIPN are in agreement with those published before in that sensory symptoms dominate and that the feet are more affected than the hands with tingling, numbness and pain as outstanding symptoms [Citation11,Citation13,Citation24]. However, in other aspects we observed several interesting and potentially important differences in our data compared with those previously reported. Thus, despite the substantially lower cumulative dose of oxaliplatin at a mean of 567 mg/m2 among our patients, the frequency of severe chronic sensory OIPN in the feet ranged 26–38%, clearly different from that observed for patients treated with fluoropyrimidine alone (), and oxaliplatin was also associated with impaired motor function although at lower frequency and intensity. In addition, we observed no obvious differences in chronic type OIPN scores in relation to patient gender, age, type of regimen or cumulated dose of oxaliplatin, although with respect to the latter individual patient treatment adjustment makes firm conclusions difficult. Furthermore, and very problematic from the patient point of view, there were also no clear-cut signs of neuropathy improvement by time since the stop of treatment. We thus conclude that despite a careful use of oxaliplatin with only 55% of planned dose given, patients in our adjuvant treatment setting are at substantial risk of contracting significant and very long-term difficult to predict neuropathy, mainly from the feet.
A study from Denmark, a country a bit similar to Sweden when it comes to seasonal differences in temperature, clearly showed much higher need for dose reductions of oxaliplatin for acute type OIPN during winter compared with summer, indicating a temperature effect on oxaliplatin tolerance [Citation25]. This is in line with our clinical experience regarding acute OIPN and such temperature effect is suggested to explain the comparatively low cumulative oxaliplatin dose among our patients compared with what has been reported from other countries, although routines and principles for assessment and handling of adverse effects during treatment might also differ. We now suggest that there is a similar temperature effect also for the severity of chronic type OIPN and, thus, that the subarctic climate in Sweden at least partly explains our findings of more severe chronic type OIPN than previously reported. In this context, it could be observed that our data collection was made during Swedish winter. However, the method used for assessment has impact on the frequency and severity of neuropathy reported [Citation8,Citation26] and it might also be that cultural differences affect patient willingness to report adverse effects.
We tried to analyze a possible impact of outside temperature a bit further by dividing our patients treated with oxaliplatin in to those having most of their first half treatment period during the cold (October–March; winter group) or warm (April–September; summer group) season. The summer group had 10% higher cumulative oxaliplatin dose and a clear but statistically non-significant trend for higher scores, such as more symptoms, for tingling (52.5 vs. 36.5 for summer and winter groups, respectively; p = 0.077) and numbness (44.4 vs. 33.3; p = 0.268) in their feet. This may suggest a positive relationship between total amount of oxaliplatin given and chronic OIPN or may be due to chance, but argues against a higher risk for chronic OIPN if oxaliplatin is administered during winter.
Most studies on chronic type OIPN have focused on the neuropathy alone and have unfortunately not assessed the impact on QoL. In the Dutch retrospective population-based study with long-term follow-up no statistically significant differences in the EORTC QLQ-C30 questionnaire function or symptom scores between patients treated with oxaliplatin or fluoropyrimidine alone were observed [Citation11]. In contrast, we observed statistically significant and seemingly clinically relevant differences between these groups in role and social functioning, nausea/vomiting as well as in financial problems and in almost every function and symptom score, patients treated with oxaliplatin reported more problems than those treated with fluoropyrimidine alone. Although not a proof, a causal relationship between QoL and OIPN is suggested by the worse OIPN reported by our compared to the Dutch patients and by the finding among the latter patients of worse QoL among patients with high compared with low neurosensory scores [Citation11]. Thus, we conclude that our patients are at substantial risk of contracting long-term neuropathy that impacts QoL following adjuvant chemotherapy with oxaliplatin.
This study has strengths and weaknesses. Its strengths are that it was performed in a country with a climate that allowed for detection of a climate influence on tolerance to oxaliplatin, included the majority of patients treated at our center during the study period and used established questionnaires for assessment of not only patient-reported OIPN, but also of QoL. Importantly for interpretation of the data, we compared oxaliplatin-treated patients with those treated with fluoropyrimidine alone and partly also with neuropathy in healthy controls. We were able to perform clinically relevant subgroup analyses for the importance of, for example, patient age, regimen, time and oxaliplatin dose for the severity and duration of OIPN. We observed the established pattern of dominating sensory symptoms from toes/feet which could be considered to validate our patient representativity and data collection, making it reasonable to believe that also data on QoL and the more detailed data on OIPN are valid.
An obvious weakness of the study is that the number of patients included is small, especially making the subgroup analyses of interest hazardous from a statistical point of view. Furthermore, it cannot be excluded that patients having had oxaliplatin-containing adjuvant treatment were biased towards reporting several and more severe symptoms as they have repeatedly been made aware from healthcare prior to start of and during treatment about the problems with OIPN, not the least as the patients are routinely asked about such symptoms prior to start of each new treatment cycle. Furthermore, the oxaliplatin group of patients had more advanced disease with higher risk for disease recurrence than patients treated with fluoropyrimidine, and it might be that this information forwarded to the patients could affect reported QoL. However, the oxaliplatin group was younger and is thus expected to have better QoL [Citation27].
We also did not collect data on, for example, socioeconomic factors and concomitant medication that might affect QoL and risk for development of neuropathy and were, thus, not able to adjust for such differences. However, one would expect the oxaliplatin group being younger and considered fit for the more intense oxaliplatin-containing adjuvant treatment to have better such characteristics, making the observed differences in QoL and OIPN even more notable. Thus, although it cannot be excluded that various types of bias might have contributed to the observed differences between the treatment groups, such impact on study outcome is reasonably small.
We conclude from the present study that oxaliplatin added to a fluoropyrimidine for adjuvant treatment of CRC in a country with subarctic climate is frequently associated with long-term, seemingly chronic adverse effects in peripheral nerve function, dominated by sensory symptoms in toes/feet, and that this is associated with clinically relevant impairment of QoL. This knowledge should be taken into account when patients are informed about the benefit and risks from including oxaliplatin in the adjuvant treatment.
Acknowledgments
The support from the Clinical Research and Development Unit, Department of Oncology, University Hospital, Uppsala, is gratefully acknowledged for patient identification and help with questionnaire distribution. Clinical Research Center Sörmland, Uppsala University, provided financial support for research to M. Stefansson.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Böckelman C, Engelmann BE, Kaprio T, et al. Risk of recurrence in patients with colon cancer stage II and III: a systematic review and meta-analysis of recent literature. Acta Oncol 2015;54:5–16.
- Påhlman L, Hohenberger W, Sugihara K, et al. Should the benefit of adjuvant chemotherapy in colon cancer be re-evaluated? J Clin Oncol 2016;34:1297–9.
- Gustavsson B, Carlsson G, Machover D, et al. A review of the evolution of systemic chemotherapy in the management of colorectal cancer. Clin Colorectal Cancer 2015;14:1–10.
- André T, Boni C, Moundedji-Boudiaf L, et al. Oxaliplatin, Fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Eng J Med 2004;350:2343–51.
- Kuebler JP, Wieand HS, ÓConnel MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol 2007;25:2198–204.
- Haller D, Tabernero J, Maroun JA, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol 2011;29:1465–71.
- Sereno M, Gutiérrez-Gutiérrez G, Gómez-Raposo C, et al. Oxaliplatin induced-neuropathy in digestive tumors. Crit Rev Oncol Hematol 2014;89:166–78.
- Ewertz M, Qvortrup C, Eckhoff L. Chemotherapy-induced peripheral neuropathy in patients treated with taxanes and platinum derivatives. Acta Oncol 2015;54:587–91.
- Land SR, Kopec JA, Cecchini RS, et al. Neurotoxicity from oxaliplatin combined with weekly bolus fluorouracil and leukovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: NSABP C-07. J Clin Oncol 2007;25:2205–11.
- Beijers AJM, Mols F, Vreugdenhil G. A systematic review on chronic oxaliplatin-induced peripheral neuropathy and the relation with oxaliplatin administration. Support Care Cancer 2014;22:1999–2007.
- Mols F, Beijers T, Lemmens V, et al. Chemotherapy-induced neuropathy and its association with quality of life among 2- to 11-year colorectal cancer survivors: results from the population-based PROFILES registry. J Clin Oncol 2013;31:2699–707.
- Storey DJ, Sakala M, McLean CM, et al. Capecitabine combined with oxaliplatin (CapOx) in clinical practice: how significant is peripheral neuropathy. Ann Oncol 2010;21:1657–61.
- Kidwell KM, Yothers G, Ganz PA, et al. Long-term neurotoxicity effects of oxaliplatin added to fluorouracil and leucovorin as adjuvant therapy for colon cancer: results from National surgical adjuvant breast and bowel project trials C-07 and LTS-01. Cancer 2012;118:5614–22.
- Postma TJ, Aaronson NK, Heimans JJ, et al. The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: the QLQ-CIPN20. Eur J Cancer 2005;41:1135–9.
- Lavoie Smith EM, Barton DL, Qin R, et al. Assessing patient-reported peripheral neuropathy: the reliability and validity of the European organization for research and treatment of cancer QLQ-CIPN20 questionnaire. Qual Life Res 2013;22:2787–99.
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Ca Inst 1993;85:365–76.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83.
- Fayers PM, Aaronson NK, Bjordal K, et al. The EORTC QLQ-C30 scoring manual. 3rd ed. European Organisation for Research and Treatment of Cancer: Brussels, 2001.
- Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med. Care 2003;41:582–92.
- Singer MA, Vernino SA, Wolfe GI. Idiopathic neuropathy: new paradigms, new promise. J Peripher Nerv Syst 2012;17S:43–9.
- Andre T, De Gramont A, Vernerey D, et al. Adjuvant fluorouracil leucovorin and oxaliplatin in stage II to III colon cancer: updated 10-year survival and outcomes according to BRAF mutation and mismatch repair status of the MOSAIC study. J Clin Oncol 2015;33:4176–87.
- Beijers AJM, Mols F, Tjan-Heijnen VCG, et al. Peripheral neuropathy in colorectal cancer survivors: the influence of oxaliplatin administration. Results from the population-based PROFILES registry. Acta Oncol 2015;54:463–9.
- Argyriou AA, Velasco R, Briani C, et al. Peripheral neurotoxicity of oxaliplatin in combination with 5-fluorouracil (FOLFOX) or capecitabine (XELOX): a prospective evaluation of 150 colorectal cancer patients. Ann Oncol 2012;23:3116–22.
- Pachman DR, Qin R, Seisler DK, et al. Clinical course of oxaliplatin-induced neuropathy: results from the randomized phase III trial N08CB (Alliance). J Clin Oncol 2015;33:3416–22.
- Altaf R, Brixen Lund A, Kristensen B, et al. Incidence of cold-induced peripheral neuropathy and dose modification of adjuvant oxaliplatin-based chemotherapy for patients with colorectal cancer. Oncology 2014;87:167–72.
- Alberti P, Rossi E, Cornblath DR, et al. Physician-assessed and patient-reported outcome measures in chemotherapy-induced sensory peripheral neurotoxicity: two sides of the same coin. Ann Oncol 2014;25:257–64.
- Derogar M, Van der Schaaf M, Lagergren P. Reference values for the EORTC QLQ-C30 quality of life questionnaire in a random sample of the Swedish population. Acta Oncol 2012;51:10–16.
