Abstract
Background: Patients diagnosed with chronic lymphocytic leukemia (CLL) are usually elderly and frequently have a number of comorbidities. Health-related quality of life (HRQoL) for these patients is of utmost importance and should be taken into consideration when assessing new treatment options. The combination of ofatumumab with chlorambucil has shown longer progression-free survival compared with chlorambucil alone. In this study, we aim to assess how this treatment combination affects patients’ health-related quality of life and patient-reported symptoms.
Material and methods: In this open-label phase III trial, patients with previously untreated CLL for whom fludarabine-based treatment was contra-indicated, were randomized 1:1 to receive oral chlorambucil (10 mg/m2) on Days 1–7 of a 28-day treatment cycle or to receive chlorambucil by this schedule plus intravenous ofatumumab (Cycle 1: 300 mg on Day 1 and 1000 mg on Day 8; subsequent cycles: 1000 mg Day 1) for 3–12 cycles. The EORTC QLQ-C30 and QLQ-CLL16 questionnaires were administered to patients before and during treatment, in follow-up and at the time of disease progression. The primary specified patient-reported outcomes were HRQoL and fatigue.
Results: Patient-reported improvements from baseline in Global Health Status (GHS)/HRQoL scores and fatigue scores were recorded during treatment with both chlorambucil monotherapy and ofatumumab in combination with chlorambucil. There were no significant differences between the two treatment arms for GHS/HRQoL (p = 0.667) or fatigue (p = 0.103). Following treatment, numerical improvements to GHS/HRQoL and fatigue scores were reported, with no significant differences between the two treatment arms.
Conclusion: Small but detectable improvements in patients’ quality of life were reported as a result of treatment. The addition of ofatumumab to chlorambucil did not negatively impact HRQoL. Quality of life was maintained in the months following treatment.
Chronic lymphocytic leukemia (CLL) is the most common form of adult leukemia in the Western world, with an annual incidence of 4.5/100 000 [Citation1]. Most patients are over 65 years of age when they are diagnosed, with the median age at diagnosis being 71 years. CLL may initially be asymptomatic and treatment is only initiated when patients meet the International Workshop on Chronic Lymphocytic Leukemia (iwCLL) criteria for active disease [Citation2]. A number of different prognostic factors have been identified including disease stage [Citation3,Citation4], genomic aberrations [Citation5] (including 17p deletion), TP53 and IGHV mutation, and overexpression of CD38 or ZAP70. These and other factors, such as fitness and/or comorbidity, determine the choice of therapy [Citation6,Citation7].
CLL remains incurable, with a profile characterized by periods of relapse and remission, and most patients survive for several years. As an incurable disease, the health-related quality of life (HRQoL) for patients following diagnosis and during/post-treatment is of great importance. A large survey of patients with CLL showed how the disease has a marked effect on patients’ HRQoL as a result of disease-specific characteristics, comorbidities, and fatigue [Citation8]. The majority of patients diagnosed with CLL is elderly and suffer from comorbidities, which tend to progressively increase with age [Citation9]. These patients may be more likely to have a somewhat lower HRQoL to begin with, compared with the general population. To date, treatment of CLL has yielded modest changes in HRQoL [Citation10,Citation11].
Ofatumumab is a human monoclonal antibody (mAb) that binds to a membrane-proximal epitope of CD20, distinct from the other anti-CD20 mAbs [Citation12]. This results in a stronger binding to CD20 and increased target cell death, via complement-dependent cytotoxicity, compared with rituximab, especially in low CD20-expressing cells, such as those found in patients with CLL [Citation12]. Ofatumumab is currently approved for the treatment of patients with CLL refractory to fludarabine and alemtuzumab and in combination with chlorambucil or bendamustine for those patients who are untreated and ineligible for fludarabine-based therapy.
The approval of ofatumumab in previously untreated patients was based on data from the Phase III COMPLEMENT 1 trial investigating the clinical safety and efficacy of ofatumumab combined with chlorambucil (O + CHL) compared with chlorambucil alone (CHL) in patients with previously untreated CLL, and for whom fludarabine was not considered appropriate due to older age or the presence of comorbidities. O + CHL demonstrated significantly longer progression-free survival (PFS) as assessed by an independent review committee (IRC) compared with CHL (22.4 vs. 13.1 months; hazard ratio = 0.57; p < 0.0001). O + CHL exhibited a manageable safety profile. Clinically meaningful improvements in secondary endpoints, including response rate, time to next therapy, duration of response, and minimal residual disease were also reported for O + CHL vs. CHL [Citation13].
Here, we report patient-reported outcomes (PROs), including HRQoL parameters and patient-reported symptoms, from the COMPLEMENT 1 study.
Methods
Patients and study design
Patient eligibility and study design have been described previously [Citation13]. Briefly, adult patients with active previously untreated CLL for whom fludarabine treatment was inappropriate were enrolled. Patients were randomized 1:1 to O + CHL or CHL arms; randomization was stratified by age, disease stage, and Eastern Cooperative Oncology Group (ECOG) performance status. Oral CHL was given at a dose of 10 mg/m2 per day on Days 1–7 of each 28-day cycle in both treatment groups. Ofatumumab was given intravenously at a fixed dose of 300 mg on the first day of Cycle 1 and 1000 mg on Day 8 of Cycle 1, and Day 1 of subsequent cycles. Treatment was received in 28-day cycles for a minimum of three cycles until best response or a maximum of 12 cycles. The institutional review board or ethics committee of each participating institution approved the study protocol. Each patient provided written informed consent before enrollment and the study was conducted according to the ethical principles of the Declaration of Helsinki.
Assessment of health-related quality of life and patient-reported outcomes
PRO measures included the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30) version 3 [Citation14] and the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Chronic Lymphocytic Leukemia 16-item module (EORTC QLQ-CLL16) [Citation15].
In the EORTC QLQ-C30, 30 items are scored in a range of different domains including: physical functioning, role functioning, emotional functioning, cognitive functioning, social functioning, pain, fatigue, nausea and vomiting, insomnia, loss of appetite, constipation, diarrhea, dyspnea, financial difficulties, and Global Health Status (GHS)/quality of life. Scores for each domain are expressed on a 0–100 scale, with 0 being worst and 100 best for the functional and GHS/HRQoL domain, and 0 being best (fewer symptoms) and 100 being worst (more symptoms) for the individual symptom scores. This questionnaire has been used in several recent studies of patients with CLL [Citation10,Citation16,Citation17]. The EORTC QLQ-CLL16 is intended as a supplementary module to the EORTC QLQ-C30. Sixteen items are scored in the following domains: disease and treatment effects, fatigue, infection, social problems, and future health. Scores for each domain are expressed on a 0–100 scale as described for the EORTC QLQ-C30.
An additional scoring approach termed patient-reported constitutional symptoms (B-symptoms) score was evaluated based on the items that reflect B symptoms from the QLQ-C30 and QLQ-CLL16 module, namely: need to rest, feeling weak, tiredness, weight loss, temperature changes, night sweats, and skin problems.
Patients were asked to complete the questionnaires at screening and every three months during treatment (Day 1 of Cycles 4, 7, and 10). The baseline score was defined as the score from the screening visit. Patients who withdrew from the study were asked to complete the questionnaires at the withdrawal visit and those patients who progressed completed the questionnaires at disease progression. Patients were also administered the questionnaires at scheduled follow-up visits (1, 3, 6, 9, and 12 months from last treatment). The baseline score for these post-treatment assessments was the last on-treatment score, to investigate how HRQoL and symptoms changed following treatment. A 12-month follow-up was considered an appropriate assessment period for this study, as the number of patients completing questionnaires beyond this point was low.
The patient-reported endpoints that were pre-specified as part of the study protocol were changes from the respective baselines during and post-treatment and treatment differences in the two arms for the GHS/HRQoL score of the EORTC QLQ-C30 and the fatigue score of the EORTC QLQ-CLL16. Other domains of the EORTC QLQ-C30 and EORTC QLQ-CLL16 were analyzed for exploratory purposes.
For treatment comparisons, analysis of covariance (ANCOVA) adjusted for baseline score, age group, Binet stage, and ECOG performance status was applied, using mixed-model repeated measures (MMRM) with intercept, time, treatment, baseline score by time interaction, and treatment by time interaction. A post hoc analysis compared changes from baseline scores for patients who responded to therapy with those who did not, regardless of the therapy received. In addition, a post hoc analysis was performed to test for differences in HRQoL according to baseline characteristics of age (<65 vs. ≥65), Binet stage, and comorbidities (<2 vs. ≥2), regardless of therapies received.
Results
Study population, treatment, and efficacy/safety outcomes
The patient population has been described previously alongside efficacy and safety results [Citation13]. Briefly, patients (N = 447) were randomly assigned to O + CHL (n = 221) or CHL (n = 226). Treatment groups were well balanced with a median age of 69 years and most [n = 391 (87%)] patients reported at least one comorbidity. Patients received a median of six treatment cycles. The primary efficacy endpoint of PFS was met, and the median PFS was significantly longer with O + CHL compared with CHL (22.4 vs. 13.1 months; hazard ratio = 0.57; p < 0.0001); secondary efficacy results also favored O + CHL. Safety results showed a manageable side-effect profile [Citation13].
Patient-reported outcomes
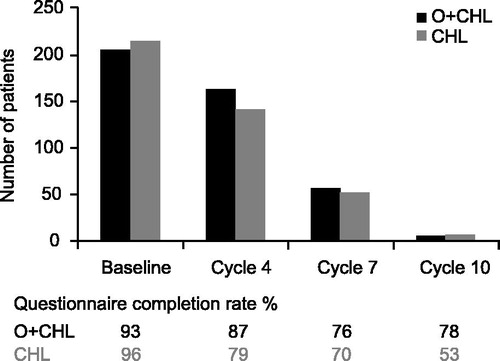
In total, 428 of 447 intent-to-treat patients (96%) completed at least one HRQoL assessment. Responses at the screening visit were similar for both treatment arms as determined by the EORTC QLQ-C30 and EORTC QLQ-CLL16. Mean baseline scores for the GHS domain of the EORTC QLQ-C30 were 65.5 for patients in the CHL arm and 63.1 for patients in the O + CHL arm, with 100 being the optimal score. The mean baseline scores at screening for the fatigue scale (EORTC QLQ-CLL16) were 29.2 in the CHL arm and 27.1 in the O + CHL arm, with 0 being the optimal score on a scale of 0–100. As per protocol, treatment was stopped once best response was achieved, resulting in two-thirds of patients stopping after six cycles of treatment. Consequently, fewer patients were on treatment after Cycle 6 to complete questionnaires and patients remaining on treatment at later time points comprised those patients who might not have yet reached maximum response (). Cycle 10 had too few patients responding to questionnaires to yield useful results (n = 15 for HRQoL).
Patient-reported outcomes during treatment
The GHS/HRQoL indicated that patients had improvements greater than the clinically relevant minimally important difference of five points [Citation18] for the CHL arm (Cycle 7) and for the O + CHL arm (Cycles 4 and 7) (). The fatigue score (EORTC QLQ-CLL16) indicated that patients had improvements from baseline in both arms at Cycles 4 and 7 ().
Figure 2. Change from baseline in Global Health Status of the EORTC QLQ-C30 for patients during the treatment period. Positive change indicates improved HRQoL.
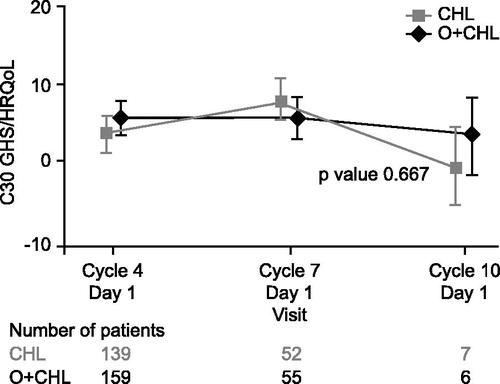
Figure 3. Change from baseline in fatigue score of the EORTC QLQ-CLL16 for patients during the treatment period. Negative change indicates improvement in symptoms.
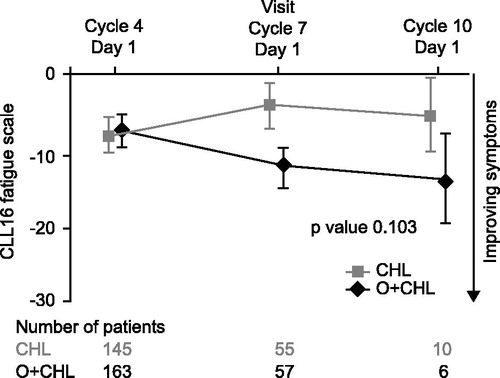
ANCOVA using MMRM results showed that there was no significant difference in change from baseline in GHS/HRQoL (p = 0.667; ) or fatigue (p = 0.103; ) between the two treatment arms during the treatment period.
The post hoc ANCOVA analysis comparing responders with non-responders, regardless of therapy, revealed no differences in the change from baseline at Cycle 4 of three key domains: GHS/HRQoL (p = 0.97), fatigue (p = 0.40), and B symptoms (p = 0.88) (). However, non-responders were less likely to complete the questionnaires (completion rate of 33% for non-responder vs. 79–84% for responders), possibly biasing this analysis.
Table 1. ANCOVA for change from baseline in GHS/HRQoL and B symptoms at Cycle 4 for patients during the treatment period, regardless of treatment arm.
There were significant improvements from baseline (p < 0.05) in the emotional functioning score of the EORTC QLQ-C30, the infection score of the EORTC QLQ-CLL16, and the B-symptom score observed in the O + CHL arm ().
Table 2. MMRM analysis for the change from baseline in B-symptom score, EORTC QLQ-CLL16 infection score, and EORTC QLQ-C30 emotional functioning for patients during the treatment period.
Patient-reported outcomes post-treatment
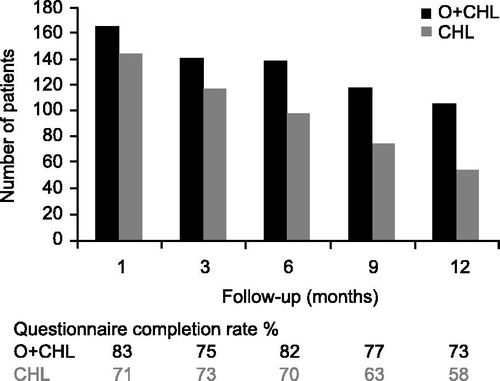
As no questionnaires were completed after progression, follow-up data only represented the period between treatment completion and progression. Follow-up data were more complete in the O + CHL arm but declined in both arms over time, likely related to progression or the start of alternative CLL therapy in the absence of documented progression. This occurred at earlier time points in the CHL arm (). GHS/HRQoL indicated that patients had positive changes from their last on-treatment visit in both arms at all time points; however, none of these differences exceeded the clinically relevant threshold of a five-point change [Citation18] (). The fatigue score indicated that patients had improvements from their last on-treatment visit in both treatment arms at all time points except for Month 12 in the O + CHL arm (). Results for other domains of the EORTC QLQ-C30 and QLQ-CLL16, as well as B-symptom score, were similar in showing numerical improvements in both arms post-treatment, for example six months following treatment the mean change from the last on-treatment visit for B-symptom score was −2.33 for the CHL arm and −1.20 for the O + CHL arm, where a negative change indicates improvement in symptoms. Therefore, results indicate that patients in both treatment arms on average maintained or increased the benefit that they had obtained during treatment.
Figure 5. Change from baseline (last on-treatment score) in Global Health Status of the EORTC QLQ-C30 for patients during post-treatment follow-up. Positive change indicates improved HRQoL.
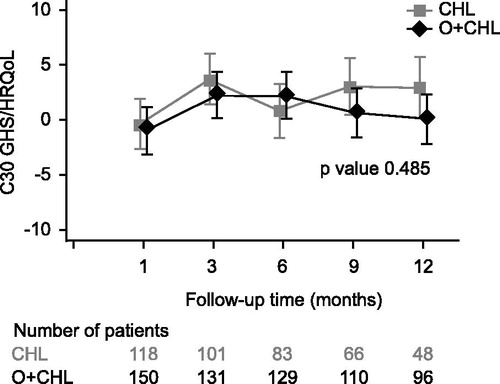
Figure 6. Change from baseline (last on-treatment score) in fatigue score of the EORTC QLQ-CLL16 for patients during post-treatment follow-up. Negative change indicates improvement in symptoms.
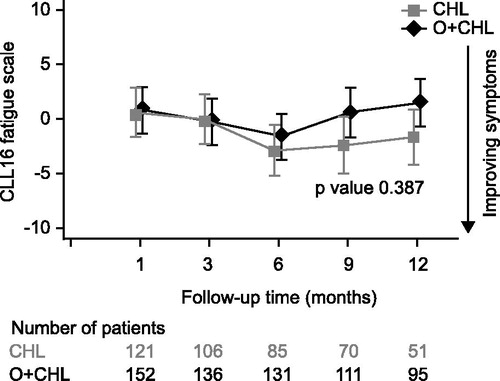
An MMRM analysis for change from last on-treatment visit showed no significant differences between the arms in the GHS/HRQoL and fatigue scores. All other domains of the EORTC QLQ-C30 and QLQ-CLL16 showed no significant differences between the two treatment arms except for physical functioning favoring the CHL arm and financial difficulties favoring the O + CHL arm (p < 0.05). There were no significant differences in B-symptom scores (p = 0.426) between the two treatment arms.
In the post hoc analysis considering HRQoL according to age (<65 vs. ≥65), Binet stage, or presence of comorbities (<2 comorbidities vs. ≥2 comorbidities), but regardless of therapy there were no differences across the subgroups either statistically or according to the clinically relevant threshold of five points difference. This was true both during treatment and during the follow-up period.
Patients with disease progression were asked to complete questionnaires at the time disease progression was assessed. For all domains including GHS/HRQoL and fatigue, patients did not report any substantive change compared with the pre-progression visit in either arm. GHS/HRQoL scores for CHL were 68.91 pre-progression and 66.49 at progression compared with 70.01 pre-progression and 70.88 at progression for the O + CHL arm. Fatigue scores for CHL were 25.09 pre-progression and 20.53 at progression compared with 21.10 at pre-progression and 21.97 at progression for the O + CHL arm.
Discussion
CLL is an incurable disease meaning that, in most cases, patients must live with their disease and periods of treatment for many years. HRQoL is therefore critically important and should be thought of as a major goal of treatment. Herein, we have evaluated the HRQoL in a group of patients for whom fludarabine was deemed an inappropriate treatment due to age and/or comorbidities. The group of patients in this COMPLEMENT 1 study was likely to already have a somewhat lower quality of life compared with the general population, due to their coexisting medical conditions.
The vast majority of patients in the study (96%) completed at least one post-baseline assessment, but the number of patients eligible to complete the questionnaires during treatment and follow-up decreased over time ( and ). Baseline scores for GHS/HRQoL were comparable with those reported from other studies of patients with CLL (GHS/HRQoL scores from previous studies have been reported as 64.5) [Citation10,Citation17]. The fact that HRQoL scores did not generally decline at progression may be due to the relatively short follow-up time for measuring HRQoL at progression, compared with the slow course of disease progression. Furthermore, progression was mostly diagnosed by an increase of lymphocyte count and would therefore not necessarily have immediately impacted the quality of life of the patient.
The addition of ofatumumab to CHL did not negatively impact HRQoL and may have improved certain aspects of patients’ well being during and after treatment. The B-symptom score, which comprised the patient scores for those domains that were preselected as B symptoms, was significantly improved in the O + CHL versus CHL arm during treatment as were the emotional functioning and infection scores.
It is important to note that the data should be interpreted with care, especially at later time points. During treatment, only patients who had not reached maximum response remained on therapy, meaning that the number of patients available to contribute data, especially after Cycle 6, decreased over the course of the treatment period. The number of patients still being assessed by Cycle 10 was too low to analyze for reliable results. During follow-up, patients only completed questionnaires until disease progression (or the start of alternate CLL therapy), which occurred sooner in the CHL arm.
The importance of HRQoL data has been highlighted by major cancer societies for some time [Citation19], however, the amount of HRQoL data in the literature from randomized controlled trials of patients with CLL is limited [Citation20], particularly in elderly patients who have been under-represented in clinical trials [Citation9]. In a study of fludarabine-based therapy in younger, fit patients with CLL, HRQoL was moderately improved by treatment and there were no differences in HRQoL between a fludarabine-only and fludarabine-plus-cyclophosphamide regimen [Citation10]. In agreement with results from our study, HRQoL was not affected by the treatment arm despite the combination arm being associated with a greater number of adverse events [Citation10,Citation16]. The lack of a treatment effect on HRQoL may be the result of a balance between receiving a more efficacious treatment and the added toxicity that this brings with it, however, confounding effects of, for example comorbidities or socioeconomic differences between the groups, may make the reasons for the similarity in HRQoL between the treatment arms more complicated to determine.
In conclusion, previously untreated patients with CLL experienced small but perceptible improvements in their HRQoL as a result of treatment and the addition of ofatumumab to CHL did not negatively impact on HRQoL. In general, patients’ HRQoL was maintained or improved upon in the months following therapy.
Acknowledgments
Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals. We thank Zoe Crossman for medical editorial assistance for this manuscript.
Disclosure statement
AM, CNC, SM, and IG were previously employed by GlaxoSmithKline and are current employees of Novartis Pharmaceuticals. They also report share ownership in both companies. PH’s institution (Department of Haematology, St James’s University Hospital, Leeds, UK) received payments from GlaxoSmithKline to cover the costs of recruitment for this study. He received personal fees for chairing a data safety monitoring board of an unrelated GlaxoSmithKline-sponsored trial and for attendance at an advisory board. AJ, KGB, JK, SG, and FO have no conflicts of interest to declare.
References
- National Cancer Institute. [Internet]. Bethesda (MD): SEER Cancer Statistics Review, 1975–2012; [cited July 2015]. Available from: http://seer.cancer.gov/csr/1975_2012/.
- Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the international workshop on chronic lymphocytic leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 2008;111:5446–56.
- Rai KR, Sawitsky A, Cronkite EP, et al. Clinical staging of chronic lymphocytic leukemia. Blood 1975;46:219–34.
- Binet JL, Auquier A, Dighiero G, et al. A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer 1981;48:198–206.
- Döhner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med 2000;343:1910–6.
- Eichhorst B, Dreyling M, Robak T, et al., ESMO Guidelines Working Group. Chronic lymphocytic leukemia: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2011;22:vi50–4.
- National Comprehensive Cancer Network. [Internet]. Fort Washington, PA: NCCN Guidelines Non-Hodgkin’s Lymphomas Version 2.2015; [cited July 2015]. Available from: www.nccn.org.
- Shanafelt TD, Bowen D, Venkat C, et al. Quality of life in chronic lymphocytic leukemia: an international survey of 1482 patients. Br J Haematol 2007;139:255–64.
- Del Giudice I, Mauro FR, Foà R. Chronic lymphocytic leukemia in less fit patients: “slow-go”. Leuk Lymphoma 2011;52:2207–16.
- Eichhorst BF, Busch R, Obwandner T, et al. Health-related quality of life in younger patients with chronic lymphocytic leukemia treated with fludarabine plus cyclophosphamide or fludarabine alone for first-line therapy: a study by the German CLL study group. J Clin Oncol 2007;25:1722–31.
- Sullivan W, Lee D, Perard R, et al. Quality of life benefits of idelalisib with rituximab for patients with previously treated chronic lymphocytic leukaemia [abstract]. Haematologica 2015;100:P214.
- Teeling JL, Mackus WJ, Wiegman LJ, et al. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J Immunol 2006;177:362–71.
- Hillmen P, Robak T, Janssens A, et al. Chlorambucil plus ofatumumab versus chlorambucil alone in previously untreated patients with chronic lymphocytic leukaemia (COMPLEMENT 1): a randomised, multicentre, open-label phase 3 trial. Lancet 2015;385:1873–83.
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–76.
- European Organisation for Research and Treatment of Cancer. [Internet]. Brussels (Belgium): Chronic lymphocytic leukaemia (QLQ-CLL16); [cited August 2015]. Available from: http://groups.eortc.be/qol/chronic-lymphocytic-leukaemia-qlq-cll16.
- Catovsky D, Richards S, Matutes E et al. Assessment of fludarabine plus cyclophosphamide for patients with chronic lymphocytic leukaemia (the LRF CLL4 Trial): a randomised controlled trial. Lancet 2007;370:230–9.
- Holzner B, Kemmler G, Kopp M, et al. Quality of life of patients with chronic lymphocytic leukemia: results of a longitudinal investigation over 1 yr. Eur J Haematol 2004;72:381–9.
- Osoba D, Rodrigues G, Myles J, et al. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 1998;16:139–44.
- Outcomes of cancer treatment for technology assessment and cancer treatment guidelines. American Society of Clinical Oncology. J Clin Oncol 1996;14:671–9.
- Efficace F, Kemmler G, Vignetti M, et al. Health-related quality of life assessment and reported outcomes in leukaemia randomised controlled trials: a systematic review to evaluate the added value in supporting clinical decision making. Eur J Cancer 2008;44:1497–506.