Abstract
Objective: To evaluate the role of interstitial pulsed dose rate brachytherapy (PDR-BT) in multimodality treatment of locally advanced primary or recurrent rectal and sigmoid cancer with high risk of microscopic incomplete resection (R1).
Methods and material: A total of 73 consecutive patients (recurrent/primary: 40/33) were treated with PDR-BT between 2001 and 2010. Patients received preoperative external beam radiotherapy (EBRT) and concomitant chemotherapy. Following resection of the tumor and the involved pelvic organs, a median of four (3–8) catheters were sutured to the tumor bed with a distance of approximately 1 cm between the catheters. A target respecting the catheters with a margin of 5 mm was contoured on computed tomography (CT) and three-dimensional (3D) dose planning with a planning aim for BT of D90 > 30 Gy, (0.6 Gy/pulse, 1 pulse/h) was performed. Previously irradiated patients (27%) underwent surgery that was directly followed by PDR-BT. Postoperative EBRT was then applied to the tumor bed 3–5 weeks after PDR-BT.
Results: A total of 23 patients (31%) received a radical resection (R0) and 45 patients (62%) received an R1 resection. Five patients (7%) received a macroscopic incomplete resection (R2). The five-year overall survival was 33%. Local control at five years was 67% for patients who received a R0 resection and 32% for patients who received an R1 resection. The five-year actuarial risk of a grade 3–4 BT-related complication was 5%.
Conclusions: Meaningful disease control and survival can be obtained at an acceptable rate of late morbidity in selected patients with locally advanced primary and recurrent rectal or sigmoid cancer using (chemo) RT, extensive surgery and PDR-BT when a high risk of an R1 resection is expected.
Approximately 10–15% of all patients with primary advanced rectal cancer (PARC) are inoperable at diagnosis [Citation1]. Despite preoperative (chemo) radiotherapy (RT) and total mesorectal excision (TME), local recurrence of rectal cancer (LRRC) is still reported in 6–10% [Citation2]. Distant metastases are the most common causes of failure accounting for 65–75% of failures [Citation3]. However, local control remains of paramount importance especially for those patients who do not obtain clear surgical margins [Citation4]. Local control is not only a prerequisite for disease-free survival but also has very important palliative value [Citation3].
Radical resection of PARC and LRRC is challenging and may often require a multimodality approach. Studies have demonstrated that centralization of rectal cancer treatment improves short- and long-term outcomes [Citation5,Citation6] and that Multidisciplinary Team (MDT) conferences improve survival. Preoperative external beam radiotherapy (EBRT) (45–50 Gy) in previously un-irradiated patients has been shown to reduce tumor size and to allow for complete resection in 50–75% of patients [Citation7]. These figures may be enhanced to some extent by concomitant chemotherapy or EBRT dose escalation [Citation4,Citation7]. However, a radical resection can still be very difficult to perform especially when the tumor involves presacral structures at the S1–S2 level and/or the pelvic side wall even after a 6–8-week rest period after EBRT to allow time for tumor regression. For patients with recurrent disease, the situation is often further complicated by a decreased radiation tolerance of the pelvis due to previous EBRT [Citation8]. Intra-operative electron beam radiotherapy (IORT), high dose rate brachytherapy (HDR-BT) or pulsed dose rate brachytherapy (PDR-BT) have been used to give an additional RT boost directly to the tumor bed to increase the chance of obtaining local control in these patients [Citation9–14].
As a strategy for patients with locally advanced primary or recurrent rectal cancer, we added PDR-BT in 2001 for patients for whom a high risk of an R1 resection was expected despite (chemo) radiation and extensive surgery. The purpose of this paper is to evaluate the clinical outcome of one of the largest series of patients treated with interstitial PDR-BT in a multidisciplinary setting at Aarhus University Hospital.
Material and methods
Patient selection and pretreatment workup
In the period between January 2001 and October 2010, we evaluated 581 patients with biopsy proven recto-sigmoid cancer. All patients were registered in an online prospective database [Citation15]. Of all of the referred patients, 148 patients were selected for palliative treatment, while 433 patients underwent surgery with curative intent. PDR-BT was used in 73 patients () with primary advanced or locally recurrent rectal and sigmoid cancer for whom microscopically radical resection (R0) was considered doubtful at a preoperative MDT conference even if extensive surgery was going to be used.
Table 1. Patient characteristics of 73 patients with locally advanced primary or recurrent rectal and sigmoid cancer with high risk of R1 resection treated with pulsed dose rate brachytherapy.
Candidates for surgery with curative intent including BT were evaluated by magnetic resonance imaging (MRI) and palpation in general anesthesia performed before any preoperative EBRT. In addition, the patients were further staged for disseminated disease by computed tomography (CT) scan of the thorax, abdomen, and pelvis or by FDG PET-CT [Citation15]. Patients with a tumor involving presacral structures at the S1–S2 level and/or pelvic side wall were selected for BT. Intra-operative frozen sections were not used in the selection process. However, if it was obvious that it was possible to obtain a R0 resection BT was omitted. Patients with disseminated disease or direct tumor invasion into the sacral bone (S1/S2) or sacral nerves were considered inoperable and were referred for palliative treatment. To exclude progression of disease in patients treated with preoperative (chemo) RT, we reevaluated all patients approximately six weeks after neoadjuvant therapy with the aim of performing surgery approximately two weeks later.
External beam radiotherapy
Previously un-irradiated patients received preoperative EBRT at the Department of Oncology. Concomitant 5FU-based chemotherapy has been added since 2002. The prescribed dose was 52 Gy in 26 fractions to the primary tumor target (CTV-T) and 46 Gy in 26 fractions to the elective target (CTV-E) covering the CTV-T as well as the uninvolved mesorectum, the internal iliac and the presacral nodes. The external iliac nodes were included in the CTV-E in case of involvement of anterior pelvic structures. Dose planning was performed based on three-dimensional (3D) CT target definition (TMS Helax, later Eclipse, Varian, Palo Alto, CA). Usually high energy photons were delivered with a simultaneous integrated boost technique using either 3D conformal radiotherapy or later intensity modulated radiotherapy. Patients referred from other regions of Denmark were treated at their regional cancer center with preoperative EBRT at doses of 52–60 Gy at 1.8–2.0 Gy/fx. Patients with limited radiation tolerance due to previous pelvic RT were operated on directly and treated with BT. These patients then received postoperative EBRT 3–5 weeks after PDR-BT. The EBRT dose in this situation was 20–25 Gy in 10–15 fractions to a limited volume, just covering the tumor bed, with no attempt of elective pelvic irradiation. Postoperative EBRT was performed at the Department of Oncology, Aarhus University Hospital. The rationale behind this treatment strategy was based on the fact that the dose of a neoadjuvant RT schedule would be severely limited due to reduced normal tissue tolerance and that the dose that could be delivered would likely be insufficient with regard to obtain significant regression. We therefore decided to rely on surgery plus BT as the initial treatment. However, the dose given by BT is by nature very heterogeneous. A very high central dose would therefore be the consequence if the full dose was given by BT. We therefore chose to give 20–25 Gy by EBRT in small doses per fractions to arrive safely at a total dose level of 50–55 Gy (BT and EBRT) which could be expected at least to control microscopic (i.e. R1) disease. Characteristics of (chemo) RT are shown in .
Table 2. Treatment characteristics for 73 patients with locally advanced primary or recurrent rectal and sigmoid cancer with high risk of R1 resection treated with external beam radiotherapy, extensive surgery and pulsed dose rate brachytherapy.
Surgery and interstitial pulsed dose rate brachytherapy
The tumor and involved organs were removed en bloc, which for some patients entailed a total pelvic exenteration (TPE) with formation of a Bricker Bladder [Citation15]. Abdominosacral resection was performed if involvement of S3/S4 was encountered. Catheters for PDR-BT (Varian, 530 mm) were sutured to the tumor bed with the blind end anchored 1–2 cm laterally to the perineal defect and the open end penetrating through the abdominal wall. The number of catheters was defined based on the width of the tumor bed with the most lateral catheters placed at the periphery of the tumor bed. The catheters were running cranio-caudal and as parallel as possible with a spacing of approximately 1 cm (). Silver seeds were used to mark the proximal and distal extensions of the tumor bed. At the end of the surgical procedure, the catheters and the perineal defect were covered with a vertical rectus abdominis myocutaneous (VRAM) flap. The histopathological staging of the specimen was performed according to the R classification proposed by Hermanek et al. [Citation16].
Figure 1. Postoperative interstitial pulsed dose rate brachytherapy (PDR-BT) in a patient with locally recurrent rectal cancer with high risk of R1 resection. (a) Catheters for brachytherapy sutured to the tumour bed. The catheters were later covered by a pediculated myocutaneous flap (rectus abdominis) preventing direct contact between the intestines and the high dose volume. (b) Treatment plan delivering 30 Gy (cyan contour) given with 0.6 Gy per pulse, one pulse per hour to the BT target (red contour). Dose planning was conducted with manual dose optimisation avoiding overlap of double dose volumes i.e., 60 Gy (yellow contour).
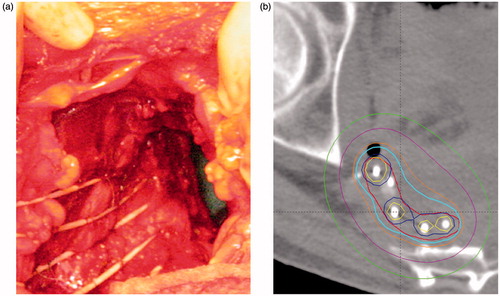
The target for PDR-BT was defined on CT using a 5 mm margin around the catheters in the area of the tumor bed supported by the preoperative MRI, the surgical findings and the silver seeds (). Marker wires were used to ensure correct reconstruction of the catheters. Manual optimization of dwell positions and dwell times was performed while avoiding overlapping double dose volumes between the catheters [Citation17]. In general, the planning aim was to achieve >30 Gy to 90% of the BT target (D90) delivered in 50 hourly pulses. However, at the beginning of the study period, the planning aim dose was 20–25 Gy in 13 patients who had received 60 Gy preoperative EBRT. Total dose of EBRT and BT was calculated as equivalent dose in 2 Gy fractions (EQD2) using the linear quadratic model with an α/β = 10 for tumor effect and α/β = 3 for organs at risk. The repair halftime was assumed to be 1.5 hours [Citation18].
Follow-up and statistics
Patients were offered follow-up at 3, 6, 12, 18, and 24 months and then yearly thereafter for up to five years after surgery at the Department of Surgery. Routine follow-up consisted of evaluation of symptoms and complications as well as a physical examination. CT and/or MR scanning were only performed if recurrence was suspected. Complications were retrospectively scored according to CTCAE V3.0 [Citation19]. The complications were divided into early (within 90 days after surgery) and late (more than 90 days after surgery) complications. Based on previous experience with IORT electrons and HDR-BT [Citation10–14] we selected soft tissue necrosis, osteonecrosis, insufficiency fracture, neuropathy and neural pain as complications that could potentially be more directly related to PDR-BT. The survival and follow-up were recorded until 4 April 2014.
The statistical analysis was performed using the STATA statistical software program (STATA®, release IC 11, StataCorp LP, TX). Survival time was calculated from the day of resection until 4 April 2014 or until death from any cause. Actuarial estimates of disease control, survival and morbidity were calculated using Kaplan-Meier statistics. For the actuarial analysis of tumor control, patients without recurrence were censored at the last follow-up or on the date of death. Local control was defined as no recurrence of disease at the site of the tumor bed. All patients with recurrent disease at the time of death were considered dead from recto-sigmoid cancer. Actuarial morbidity was analyzed as morbidity grade 1 or worse, grade 2 or worse, and grade 3 or worse. Patients were censored from the analysis of morbidity in the case of a recurrence or death. Differences in observed survival between groups were tested for statistical significance using the Wilcoxon-Breslow-Gehan test. The Fischer’s exact test was used to compare the distribution between categorical variables. Student’s t-test was used to assess whether the means between the two groups were significantly different. The data were analyzed as two independent samples from a normal distribution. The assumption of normality was checked by a Q-Q plot, and if this assumption was violated, a Wilcoxon rank sum test was used.
Results
Patient and treatment characteristics
The patient cohort was dominated by male patients with recurrent rectal cancer (). The median age was 62 (range 38–82) years. As expected, only 23 (31%) had an R0 resection, 45 (62%) had an R1 resection and five (7%) had a macroscopic incomplete resection (R2). TPE was performed in approximately half of the patients and approximately one-third had resection of the sacral bone (). The median operating time was 360 (range 165–555) min. Fifty-three (73%) previously un-irradiated patients were treated with preoperative EBRT (), whereas 16 previously irradiated patients received postoperative EBRT. Four patients did not receive any EBRT either preoperatively or postoperatively. The target volume for PDR-BT was 23 cm3 (range 2–68) using a median of four catheters (range 3–8) to cover the tumor bed (). The average D90 obtained by PDR-BT was 37 Gy (range 24–45).
Outcome
The median follow-up time was 40 months (range 2–144). At the last follow-up, 12 patients (16%) were alive without relapse. Thirty-five patients (48%) were diagnosed with a local recurrence, of which 17 patients had distant metastasis. In total, 33 patients (45%) were diagnosed with distant metastasis. The five-year overall survival was 33% (95% CI 22–44%) (). There was no difference in overall survival between patients treated for primary or recurrent disease (p = .40) with five-year survival figures of 43% (95% CI 25–60%) and 28% (95% CI 16–42%), respectively. The actuarial five-year local control rates for primary and recurrent disease were 51% (95% CI 29–69%) and 42% (95% CI 25–58%), respectively (p = .19). Local control was also similar when comparing previously irradiated and un-irradiated patients (p = 0.95). As demonstrated in , local control was significantly better (p = .02) in patients treated with 3–4 catheters (66%) compared to patients treated with 5–8 catheters (34%). There was no difference in actuarial local control for patients treated with a dose above or below the median of the D90 of PDR-BT. Similarly, we did not observe an effect when including the dose of EBRT by calculating the combined EQD2 of EBRT and BT. The five-year local control after a R0 resection was 67% (95% CI 43–83%), and it was 32% (95% CI 16–50%) after R1 resection, but this difference was not significant (p = .35) (). No patients with an R2 resection obtained local control.
Figure 2. Clinical outcome in 73 patients with locally advanced primary or recurrent rectal and sigmoid cancer with high risk of R1 resection treated with pulsed dose rate brachytherapy (PDR-BT). (a) Local control (LC) and overall survival (OS) of the entire group. (b) Local control related to the number of catheters used for brachytherapy. (c) Local control in relation to resection margin (R-stage). (d) Freedom from Grade 3, 4 and 5 overall and specific brachytherapy-related morbidity ().
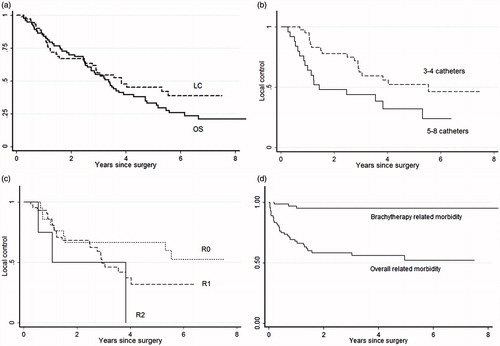
No patients died within 30 days, and only one patient died during the hospital stay (1%) due to pneumonia (grade 5 event). Sixteen patients (21%) did not experience any early or late complications. Acute toxicity was found in 32 patients (43%) and included mainly infections (). Late complications were reported in 39 patients (53%), with 21 grade 3–4 events. No patients died because of late morbidity. Bladder emptying problems were the dominant late complication and were found in 17 patients (23%). Persistent cutaneous fistulas were seen in seven patients (10%). There was no difference in the rate of severe late morbidity G3–4 between previously irradiated and un-irradiated patients (p = .64). Only three patients experienced grade 3–4 PDR-BT-associated complications in the form soft tissue necrosis and osteonecrosis, which was observed during the first year of follow-up. No additional grade 3–4 events related to PDR-BT were seen at a later time (). Neural symptoms grade 1–2 were seen within 90 days after surgery in eight patients (11%). Late neural toxicity was seen in 15 patients (21%) but was limited to grade 1–2.
Table 3. Early (within 90 days) and late complications (>90 days) retrospectively scored according to CTCv3.0 in 73 patients with locally advanced primary or recurrent rectal and sigmoid cancer with high risk of R1 resection treated with external beam radiotherapy, extensive surgery and pulsed dose rate brachytherapy.
Discussion
Considering the poor prognosis for patients with locally advanced rectal cancer following an R1 resection, our study demonstrates that extensive surgery combined with PDR-BT is feasible and results in meaningful local control and survival and, at the same time, acceptable morbidity. No differences in local control or overall survival between patients with primary and recurrent disease were found. This is in accordance with Tveit et al.’s analysis of 66 patients treated with IORT [Citation13]. Nuyttens et al. reported a significant difference in local control between primary and recurrent disease in only 37 patients (p = .042) using HDR-BT [Citation12]. Selection bias and the limited number of patients involved in all three studies are likely explain this difference. We believe that recurrent disease in itself is not a disqualification for multimodality treatment. This view is supported by the study of Tepel et al., who used a setup similar to ours and reported an overall five-year survival of 23% for recurrent colorectal cancer [Citation10].
Several studies have shown that radical resection has a significant influence on local control [Citation9–11,Citation20,Citation21]. However, like Nuyttens et al. [Citation17], we did not find a statistically significant impact when comparing patients with R0 and R1 resection. This finding could be attributed to lack of power but could also be partly attributed to an effect of PDR-BT being able to compensate for an R1 resection because, in the first two years after surgery, the local control was similar between R0 and R1 resection (). Furthermore, the five-year local control rate of 32% after R1 resection is good compared to other studies [Citation10,Citation12,Citation14,Citation22,Citation23]. This is further supported by the observation that the number of catheters significantly influenced the possibility of achieving local control. Thus, for a given dose of PDR-BT and assuming a given number of tumor clonogens per unit area, the likelihood of achieving local control in all subsections of a finite size will be less in a larger than in a smaller tumor bed because failure in just one subsection will cause overall local failure. Clinical data and radiobiological interpretations of the relationship between tumor volume and local control support this hypothesis [Citation24]. This implies that the size of the tumor bed could be as important as the resection status when discussing the indication and limitations of PDR-BT. In general, the literature reporting the results of IORT and BT in PARC or LRCC is difficult to analyze as many of the studies applied these treatments only in cases of increased risk of incomplete resection, whereas other centers used IORT or BT routinely in the presacral space regardless of the tumor topography and the expected surgical result [Citation10,Citation12,Citation14,Citation25,Citation26]. Furthermore, the definition of close or positive margins varied, and only a few studies included both primary and recurrent disease [Citation12,Citation13]. The techniques for BT, dose and fractionation in the different studies are heterogeneous, which makes direct comparisons difficult. We did not observe an effect of dose on local control over the limited range of doses applied here. With some variation, we used PDR-BT with a median D90 of 36 Gy, which delivered in 50 hourly pulses is equivalent to approximately 36 Gy given in 2 Gy per fraction (EQD2). With IORT, 10–20 Gy is usually given with 12–15 MeV electrons in one fraction directly to the tumor bed [Citation9]. Using the LQ model, this corresponds to 16.7–50 Gy EQD2 for tumor effect (α/β = 10) and 26–92 Gy for OAR (α/β = 3). So far, the IORT studies have also not been able to demonstrate a clear effect of dose escalation on local control. It is therefore unlikely that dose escalation will improve the rates of local control. IORT using HDR-BT has resulted in improved target coverage and better possibilities of avoiding irradiation of normal tissue, such as intestines [Citation11]. However, the high dose rate limits the dose that can be safely delivered. With PDR, the improved target coverage of the HDR afterloading technique is combined with the radiobiological advantage of low dose rate. This viewpoint is supported by a non-randomised study showing a trend in favor of PDR compared with HDR [Citation10].
Recently, particle therapy with carbon ions has been proposed as a way to improve to local control in LRRC. However, the clinical information provided so far is either limited [Citation27] or the studies are too small with too short follow-up [Citation28] to assess whether this new treatment option is competitive. In the study by Habermehl [Citation28], the planning target volume was also considerably larger even with carbon ions (456 cm3) compared to our study with PDR-BT (23 cm3) without being able to deliver more dose to the tumor area.
Despite the extensive surgery, the postoperative mortality in our study was low and comparable with the postoperative rate of mortality for primary rectal cancer [Citation2,Citation14]. The reported postoperative morbidity varies widely [Citation2,Citation14,Citation25]. Overall, we found a high complication rate, as only 21% of patients did not experience any complications at all. However, our early complication rate is in concordance with other studies [Citation14,Citation25]. As seen in , late morbidity continued to evolve over the years, whereas the BT-associated morbidity remained stable. As opposed to studies reporting the results of IORT with electrons or HDR-BT, we did not find any G3–4 neurological complications. Considering that previous RT did not influence the chance of obtaining local control or the risk for developing severe morbidity, previous pelvic EBRT should not by itself disqualify patients from multimodality treatment.
Conclusion
For selected patients with locally advanced primary or recurrent rectal and sigmoid cancer with high risk of R1 resection, PDR-BT in combination with EBRT and extensive surgery is a reliable modality to improve outcome with a meaningful local control survival. Significantly better local control is obtained if the tumor bed is small and only requires three or four catheters. Previous RT or recurrent disease does not disqualify patients for this multimodality treatment. However, achievement of an R0 resection is still very important, the failure of which is unlikely to be overcome by dose escalation of PDR-BT.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Glimelius B. Radiotherapy in rectal cancer. Br Med Bull 2002;64:141–57.
- Palmer G, Martling A, Cedermark B, et al. A population-based study on the management and outcome in patients with locally recurrent rectal cancer. Ann Surg Oncol 2007;14:447–54.
- Farouk R, Nelson H, Gunderson LL. Aggressive multimodality treatment for locally advanced irresectable rectal cancer. Br J Surg 1997;84:741–9.
- Rodel C, Grabenbauer GG, Matzel KE, et al. Extensive surgery after high-dose preoperative chemoradiotherapy for locally advanced recurrent rectal cancer. Dis Colon Rectum 2000;43:312–9.
- Iversen LH, Harling H, Laurberg S, et al. Influence of caseload and surgical speciality on outcome following surgery for colorectal cancer: a review of evidence. Part 1: short-term outcome. Colorectal Dis 2007;9:28–37.
- Iversen LH, Harling H, Laurberg S, et al. Influence of caseload and surgical speciality on outcome following surgery for colorectal cancer: a review of evidence. Part 2: long-term outcome. Colorectal Dis 2007;9:38–46.
- Wong CS, Cummings BJ, Brierley JD, et al. Treatment of locally recurrent rectal carcinoma-results and prognostic factors. Int J Radiat Oncol Biol Phys 1998;40:427–35.
- Mohiuddin M, Marks G, Marks J. Long-term results of reirradiation for patients with recurrent rectal carcinoma. Cancer 2002;95:1144–50.
- Harrison LB, Minsky BD, Enker WE, et al. High dose rate intraoperative radiation therapy (HDR-IORT) as part of the management strategy for locally advanced primary and recurrent rectal cancer. Int J Radiat Oncol Biol Phys 1998;42:325–30.
- Tepel J, Niehoff P, Bokelmann F, et al. Feasibility and early results of interstitial intensity-modulated HDR/PDR brachytherapy (IMBT) with/without complementary external-beam radiotherapy and extended surgery in recurrent pelvic colorectal cancer. Strahlenther Onkol 2005;181:696–703.
- Alektiar KM, Zelefsky MJ, Paty PB, et al. High-dose-rate intraoperative brachytherapy for recurrent colorectal cancer. Int J Radiat Oncol Biol Phys 2000;48:219–26.
- Nuyttens JJ, Kolkman-Deurloo IK, Vermaas M, et al. High-dose-rate intraoperative radiotherapy for close or positive margins in patients with locally advanced or recurrent rectal cancer. Int J Radiat Oncol Biol Phys 2004;58:106–12.
- Tveit KM, Wiig JN, Olsen DR, et al. Combined modality treatment including intraoperative radiotherapy in locally advanced and recurrent rectal cancer. Radiother Oncol 1997;44:277–82.
- Dresen RC, Gosens MJ, Martijn H, et al. Radical resection after IORT-containing multimodality treatment is the most important determinant for outcome in patients treated for locally recurrent rectal cancer. Ann Surg Oncol 2008;15:1937–47.
- Nielsen MB, Rasmussen PC, Lindegaard JC, et al. A 10-year experience of total pelvic exenteration for primary advanced and locally recurrent rectal cancer based on a prospective database. Colorectal Dis 2012;14:1076–83.
- Hermanek P, Wiebelt H, Staimmer D, et al. Prognostic factors of rectum carcinoma–experience of the German Multicentre Study SGCRC. German Study Group Colo-Rectal Carcinoma. Tumori 1995;81:60–4.
- Tanderup K, Hellebust TP, Honore HB, et al. Dose optimisation in single plane interstitial brachytherapy. Radiother Oncol 2006;81:105–11.
- Lindegaard JC, Fokdal LU, Nielsen SK, et al. MRI-guided adaptive radiotherapy in locally advanced cervical cancer from a Nordic perspective. Acta Oncol 2013;52:1510–19.
- Cancer therapy evaluation program. Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events, Version 3.0. Internet. 2006. http://ctep.cancer.gov/protocolDevelopment/adverse_effects.htm.
- Kusters M, Valentini V, Calvo FA, et al. Results of European pooled analysis of IORT-containing multimodality treatment for locally advanced rectal cancer: adjuvant chemotherapy prevents local recurrence rather than distant metastases. Ann Oncol 2010;21:1279–84.
- Willett CG, Shellito PC, Tepper JE, et al. Intraoperative electron beam radiation therapy for recurrent locally advanced rectal or rectosigmoid carcinoma. Cancer 1991;67:1504–8.
- Nielsen M, Rasmussen P, Pedersen B, et al. Early and late outcomes of surgery for locally recurrent rectal cancer: a prospective 10-year study in the total mesorectal excision era. Ann Surg Oncol 2015;22:2677–84.
- Valentini V, Morganti AG, Gambacorta MA, et al. Preoperative hyperfractionated chemoradiation for locally recurrent rectal cancer in patients previously irradiated to the pelvis: a multicentric phase II study. Int J Radiat Oncol Biol Phys 2006;64:1129–39.
- Bentzen SM, Thames HD. Tumor volume and local control probability: clinical data and radiobiological interpretations. Int J Radiat Oncol Biol Phys 1996;36:247–51.
- Turley RS, Czito BG, Haney JC, et al. Intraoperative pelvic brachytherapy for treatment of locally advanced or recurrent colorectal cancer. Tech Coloproctol 2013;17:95–100.
- Roeder F, Treiber M, Oertel S, et al. Patterns of failure and local control after intraoperative electron boost radiotherapy to the presacral space in combination with total mesorectal excision in patients with locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 2007;67:1381–8.
- Kamada T, Tsujii H, Blakely EA, et al. Carbon ion radiotherapy in Japan: an assessment of 20 years of clinical experience. Lancet Oncol 2015;16:e93–100.
- Habermehl D, Wagner M, Ellerbrock M, et al. Reirradiation using carbon ions in patients with locally recurrent rectal cancer at HIT: first results. Ann Surg Oncol 2015;22:2068–74.
