Abstract
Background: Whole body positron emission tomography (PET)/computed tomography (CT) is a sensitive imaging technique in patients with metastatic melanoma, but its role in the follow-up of asymptomatic high-risk patients is unclear. The aim was to study the role of PET/CT as a routine surveillance imaging tool in asymptomatic high-risk patients at the early stage of follow-up combined with a sufficient follow-up over several years.
Material and methods: A total of 110 asymptomatic patients with clinically local American Joint Committee on Cancer (AJCC) stage IIB-IIIB melanoma underwent routine whole body PET/CT scanning after a mean interval of seven months after initial surgery. Clinical data were retrospectively analyzed after a median follow-up time of 4.6 years.
Results: Recurrent melanoma was detected in 45 patients (41%) and 36 (33%) died of melanoma. In 11 asymptomatic patients (10%) occult disease was detected with a single PET/CT. In seven of these patients (64%), positive PET/CT finding had major influence in treatment decisions. Four patients underwent surgical metastasectomy and two of them remained disease-free. In 34 patients (31%) PET/CT revealed no disease, but recurrence was detected at a median time of 19 months after negative PET/CT scan. In 50 patients (45%) PET/CT finding was true negative. In 15 patients (14%) scan was false positive leading to additional management or repetitive imagings.
Conclusion: A single PET/CT could detect 24% of all recurrences in asymptomatic melanoma patients at the early stage of follow-up, but an earlier detection of occult metastases did not improve survival.
Melanoma is a leading cause of all skin cancer deaths in Western countries and its incidence has increased over the past decades [Citation1,Citation2]. After a latent symptom-free period, a proportion of patients carry a risk for developing recurrence and metastases. Whenever occult disease has initially not been detected, the metastases at some stage will grow and become clinically manifest. In the clinical setting, accurate staging is important in estimating the potential risk of recurrence. The goal of the follow-up is to detect such recurrent melanoma among high-risk patients as early as possible, because in theory, there may be a potential chance for surgery or for other options, such as medical therapy with new immunotherapeutic and targeted agents [Citation3]. The outcome might be better if immunotherapy is introduced when the tumor burden is limited.
Among imaging modalities, whole body fluorine-18 fluoro-2-deoxyglucose (FDG) positron emission tomography (PET) is a potential tool to detect metastases of melanoma based on abnormal cellular glucose uptake. PET is more sensitive and specific in the detection of malignant tumors than conventional imaging studies, such as computed tomography (CT) alone [Citation4]. Disadvantages of PET include its limited ability to detect brain metastases and lesions smaller than 5 mm, its limited availability and higher costs compared with CT [Citation5]. Also, curtailed anatomical information provided by PET often renders accurate localization of metastatic lesions difficult. Over the last decade, dual modality hybrid PET/CT scanner has become a standard technique, because it is clearly superior to PET or CT alone providing accurately fused morphological and functional imaging within single examination [Citation6,Citation7].
There are four potential indications for the use of PET/CT in patients with melanoma: primary staging at the time of initial disease presentation, surveillance during follow-up in asymptomatic high-risk patients, preoperative evaluation in patients with metastatic disease who are candidates for surgery, and therapy monitoring in patients with metastatic disease undergoing non-surgical oncologic treatment. Among these strategies, PET/CT prior to surgical resection has been reported to be cost-effective also providing a small survival benefit [Citation8].
The role of imaging in the follow-up of high-risk melanoma is not clearly defined and almost all recommendations are based rather on common sense or historical practice than evidence-based guidance. Regarding the use of whole body PET/CT during follow-up in asymptomatic patients, there is no high-level evidence or even consensus guiding the follow-up protocols, optimal indications and timing of PET/CT.
In this study, the role of PET/CT was investigated as a routine surveillance imaging tool in asymptomatic high-risk patients at the early stage of follow-up combined with a sufficient follow-up over several years. The aim was to study the impact of PET on treatment decision making and on the prognosis in terms of disease-free survival (DFS) and overall survival (OS).
Material and methods
Patients
From 2004 to 2011, consecutive patients with clinically local American Joint Committee on Cancer (AJCC) stage IIB-IIC (sentinel node-negative) or IIIA-IIIB (sentinel node-positive) cutaneous melanoma were prospectively enrolled into the study database at the Department of Plastic and General Surgery, Turku University Hospital, Turku, Finland. The study protocol was approved by the Institutional Review Board of the Turku University Hospital and an informed consent was obtained of each patient.
All patients underwent sentinel node biopsy (SNB) with standard technique. Completion lymph node dissection (CLND) was performed in sentinel-positive patients. All patients underwent whole body PET/CT, which was scheduled to be performed after an interval of six months after initial surgery. The patients were excluded, if PET/CT was performed earlier than three months or later than 12 months after surgery. If no further surgery was considered, the patients were then referred for further follow-up to the Department of Oncology and Radiotherapy, Turku University Hospital. The follow-up protocol consisted of clinical examination every 3–6 months during the first five years. Routine chest x-ray and blood tests including liver chemistry were performed annually. No additional PET/CT scanning was routinely repeated if the patient remained asymptomatic and if there was no clinical suspicion of recurrent disease. The most recent follow-up information was retrospectively collected from the electronic patient records of Turku University Hospital in 2015. The cause and time of death were obtained from the patient records and from the autopsy reports.
PET/CT imaging procedure
For whole body 18F-FDG-PET/CT scan (Discovery STE or VCT, General Electric Medical Systems, Milwaukee, WI, USA) patients fasted for a minimum of six hours before the intravenous injection of 4 Mbq/kg 18F-FDG. Low-dose PET/CT (kV 120, Smart mA range 10–80) from calvarium to toes was performed after 50–60 minutes from injection. PET images were corrected for dead time, decay, and photon attenuation and were reconstructed with 128 × 128 matrix size in fully three-dimensional (3D) mode using ML-OSEM reconstruction algorithm. Imaging analysis was performed using ADW 4.5 workstation. 18F-FDG PET/CT images were analyzed visually and semiquantitatively by calculating maximum standardized uptake value (SUVmax), defined as the ratio of activity per milliliter of tissue to activity in the injected dose corrected for decay and for the patient’s body weight.
Definitions and study endpoints
The result of PET/CT was defined as true positive (TP), if metastatic disease was detected by the first scanning in an asymptomatic patient. PET/CT finding was defined as true negative (TN), if the first scanning was negative and no disease was detected during further follow-up. PET/CT result was defined as false negative (FN), if the first scanning was negative, but recurrent disease was detected during further follow-up. PET/CT finding was defined as false positive (FP), if the possibility of metastatic disease was suspected based on active foci in the scan leading to biopsy, surgical management, medical treatment, or repetitive PET scannings or other imagings. Sensitivity was calculated as TP/(TP + FN) × 100, specificity as TN/(TN + FP)/100, accuracy as TN + TP/(TN + TP + FN + FP) × 100, positive predictive value (PPV) as TP/(TP + FP) × 100, and negative predictive value (NPV) as TN/(TN + FN) × 100. The type of recurrence was defined according to the site of the first relapse. DFS was defined as the time from initial surgery to the detection of recurrent melanoma. Cancer-specific OS was defined as the time from initial melanoma treatment to the disease-specific death of metastatic melanoma. The primary endpoint of survival analyses was melanoma-related death. The secondary endpoint was the recurrence of melanoma.
Statistical analyses
In statistical analyses, categorical variables were analyzed by the χ2-test and continuous data by the non-parametric Mann-Whitney U test. The starting point for all survival analyses was the initial melanoma treatment. The cutoff point for survival analyses was 10.0 years. DFS and cancer-specific OS curves were constructed by the Kaplan-Meier method and the group differences were analyzed by the log rank test. The ticks along the curves in the survival plots represent censored observations. Deaths from other causes or unknown outcome were marked as censored observations for cancer-specific survival. A p-value of less than 0.05 was considered statistically significant.
Results
A total of 110 asymptomatic patients with AJCC stage IIB-III melanoma underwent whole body PET/CT after a median interval of seven months (mean 7; range 3–12) after initial surgery. The median follow-up time of the patients was 56 months (4.6 years). The baseline characteristics of the study patients are presented in . Overall, 45 patients (41%) had recurrence and 36 (33%) died of melanoma. In 11 asymptomatic patients (10%) occult disease was detected with a single PET/CT. In 34 patients (30%) PET/CT revealed no disease, but recurrence was detected after a median period of 19 months (mean 25; range 3–89). All of these late recurrences were detected because of clinical symptoms, not by routine imaging alone. PET/CT was however used in many of such cases, if there was clinical suspicion of metastatic disease. The follow-up events of study patients, separatively according to sentinel node status (AJCC stage IIB-C vs. stage IIIA-B), are presented in . The Kaplan-Meier curves of DFS between sentinel-negative and -positive patients are presented in . Sentinel-negative patients with AJCC stage IIB-IIC melanoma had shorter DFS than sentinel-positive patients with stage IIIA-IIIB melanoma, but the difference was not statistically significant by the log rank test. In 50 patients (45%) PET/CT finding was TN and no recurrence was detected during follow-up. In 15 patients (14%) PET/CT scan was FP. Five of them had additional occult primary cancer and 10 patients had benign cause verified by surgery, biopsy or repeated imaging studies. Overall, sensitivity for the whole follow-up was 25.0%, specificity 76.9%, PPV 42.3%, NPV 59.5%, and accuracy 55.5%. These values were also calculated separatively at different follow-up periods after PET/CT ().
Figure 1. Kaplan-Meier curves of disease-free survival between AJCC stage IIB–IIC (sentinel-negative) and IIIA–IIIB (sentinel-positive) patients. The differences were not statistically significant.
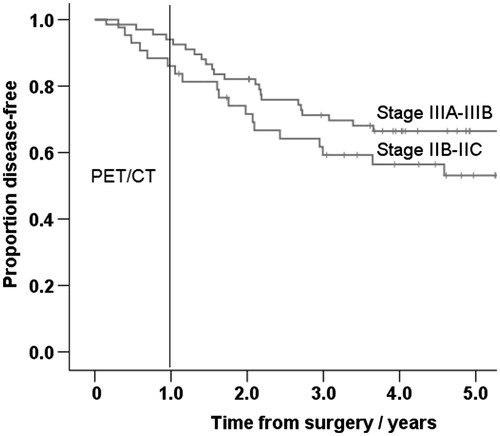
Table 1. Clinical and histopathological characteristics of 110 study patients.
Table 2. Follow-up events and final status of 110 study patients.
Table 3. Sensitivity, specificity, accuracy, PPV, and NPV of PET/CT to detect occult metastasis in 110 asymptomatic AJCC stage IIB-IIIC melanoma patients at different follow-up time points after PET/CT.
In the subgroup of patients with TP PET/CT, four of 11 patients (36%) underwent surgical metastasectomy and in the subgroup of FN PET/CT, nine of 34 patients (26%) had surgery. Four patients (two patients in both subgroups) remained disease-free 3.9, 5.7, 7.8, and 8.9 years after surgery. In the TP group, six of 11 patients (55%) received chemotherapy, immunotherapy or radiotherapy. In the FN group, 22 of 34 patients (65%) received medical therapy or radiotherapy. There were no statistically significant differences in DFS or cancer-specific OS between these subgroups.
Discussion
In this study, 41% of patients with AJCC stage IIB-IIIB melanoma had recurrent disease during a median follow-up time of 4.7 years. A total of 24% of these recurrences (10% of all patients) were detected in asymptomatic patients with a single whole body PET/CT after an interval of seven months after initial surgery.
In many previous studies, the sensitivity of PET/CT has been reported to be superior. An overall sensitivity of 89.4% in detecting systemic metastases in patients with stage III melanoma was calculated in a recent review [Citation9]. However, the definition of sensitivity needs a deeper discussion in this context. There is no clear rule on how many and how frequent scannings or how long follow-up are needed to estimate sensitivity, if PET/CT is used for screening purposes. Recurrent melanoma cannot be ruled out by a one-time imaging, because patients with high-risk melanoma have a life-long risk of recurrent disease and, therefore, the sensitivity of any surveillance method correlates with the length of follow-up. In this study, the sensitivity of PET/CT to detect occult melanoma was 78.6%, if the follow-up time had a cutoff at six months. In contrast, at five years, the sensitivity of a single scanning to predict later recurrence decreased to 29.5%. The optimal length of the follow-up is not certain, as each patient carries an unpredictable and individual course of the disease progression.
There remains a high risk for later recurrence even if the initial scan is negative. PET/CT is a sensitive diagnostic tool only when repeated several times during follow-up. The Danish Health Authority recommends repeated PET/CT scans for high-risk melanoma patients (stage III) at 6, 12, 24, and 36 months after surgery [Citation10]. Such an active approach seems to be a global trend and the use of PET/CT is increasing. According to the tumor registry of the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database, the use of PET scanning for melanoma increased from 4.2% in 2000 to 12.1% in 2007 in the US [Citation11]. The availability of PET/CT is also expanding. In Europe, 551 PET/CT cameras produced about 900 000 examinations, amounting to 1200 examinations per million inhabitants in 2012 [Citation12]. However, the decision to order staging studies should also take into account the morbidity associated with further workup, the amount of radiation exposure, costs, and an estimate whether the findings will prompt changes in patient management. The potential benefit of repetitive routine PET/CT’s in terms of survival or cost-effectiveness has not been investigated in large randomized studies.
The specificity of PET/CT is less than sensitivity, dependent on the timeframe of follow-up. It varied between 77.9% and 84.4% in this study. However, FP findings are a major problem because they are inevitably followed by additional management leading to potential morbidity and extra costs. In this study, 58% of PET/CT findings were FP causing further workup. Two of these 15 patients underwent unnecessary surgery because of suspicious findings: one patient had open thoracotomy and another had radical axillary lymph node dissection. Additional occult primary cancer was found in five patients (4%): three thyroid cancers, one breast cancer, and one kidney cancer. They were all operated properly, but universal cancer screening was not the purpose of PET/CT de facto and, therefore, these cases were regarded as FP findings in this study context. Furthermore, two patients had colon adenomas, two had benign thyroid nodules, one had adrenal gland adenoma, and three patients had unspecific findings leading to repetitive imaging controls unless the initial PET/CT finding was judged to be benign. In some other studies the FP rate has been reported to be as high as 85% [Citation13].
Patient selection and the definition of high-risk patient also need special attention. Baker et al. included only sentinel-positive stage III patients in their study and concluded that PET/CT had minimal utility in identifying unsuspected recurrence with short follow-up [Citation14]. Horn et al. also included only stage III patients and found 12% of PET scans to detect clinically occult melanoma metastasis [Citation15]. Some consensus guidelines do not recommend routine imaging for patients with clinically node-negative melanoma [Citation16]. However, we demonstrated in this study that sentinel-negative patients with AJCC stage IIB-IIC melanoma had even worse prognosis than those with stage IIIA-IIIB micrometastatic nodal involvement. This observation is in accordance with previous studies [Citation17]. Also, the sensitivity of PET/CT in detecting occult disease was better in sentinel-negative stage IIB-IIC patients. PET/CT detected seven TP relapses (16%) in stage II patients, whereas only four (6%) in stage III patients, respectively. Obviously, all patients with ulcerated intermediate thickness (>2.0 mm) melanoma (category T3b) or with any thick melanoma (category T4a-b) should be regarded as high-risk patients.
If PET/CT is considered as a routine imaging method during follow-up, attention has to be paid to its impact on treatment decision [Citation18]. It has been estimated that PET/CT may lead to altered management in 10–19% of cancer patients [Citation19]. In a retrospective study by Schüle et al., the results of PET/CT led to therapy change in 17–59% in patients with stage III/IV melanoma [Citation20]. We have earlier reported an upstaging in 20% of asymptomatic stage IIB-IIIB patients and this upstaging influenced management in every case [Citation21]. However, in the current study with a different inclusion protocol, upstaging was detected only in 11 of 110 patients (10%), of which eight patients either underwent surgical metastasectomy and/or received chemotherapy, immunotherapy, or targeted drugs. Two patients have remained disease-free after the surgical resection: one obese patient had non-palpable subcutaneous metastasis in the trunk and another patient had regional lymph node metastasis, which was initially missed because of unsuccessful lymphoscintigraphy and SNB.
Our study has limitations. The number of patients is too low to demonstrate any survival benefit of PET/CT. Melanoma-related death was defined as the primary endpoint in the initial study protocol, based on the relatively long follow-up time (median, 4.6 years), which was assumed to be sufficient to estimate survival. However, the focus on the value of a single PET/CT is to predict recurrence. Because this study is retrospective, there may be incomplete reporting of patient data and inconsistency in surveillance protocols. There may also be some inconsistency in the verification of borderline PET/CT findings with a low or moderate level of suspicion, especially if there was a limited chance to histologic biopsy. We are also aware that the accuracy of PET/CT is difficult to analyze on the basis of a single-time scanning and a more extensive protocol with repeated scans will be more informative. Late relapses can be detected only by later scans. However, we underline that our study avoided referral bias, because all patients were asymptomatic and were referred consecutively to PET/CT without any clinical suspicion of recurrence. In a recent review, this referral bias was found to be a confounding factor in many corresponding studies, because only a few of them included clinically asymptomatic patients [Citation22].
In conclusion, routine PET/CT found 24% of all recurrences in asymptomatic patients with high-risk melanoma, when it was used as a single-time imaging at the early stage of follow-up. Over half of all positive PET/CT scans were FP. The results of this study do not demonstrate any survival benefit of PET/CT and do not thus support the routine use of PET/CT. A prospective randomized trial needs to be conducted to answer the question whether imaging during follow-up improves survival for asymptomatic high-risk melanoma patients.
Disclosure statement
The authors declare that there is no conflict of interest.
References
- Tryggvadottir L, Gislum M, Hakulinen T, et al. Trends in the survival of patients diagnosed with malignant melanoma of the skin in the Nordic countries 1964-2003 followed up to the end of 2006. Acta Oncol 2010;49:665–72.
- Leiter U, Eigentler T, Garbe C. Epidemiology of skin cancer. Adv Exp Med Biol 2014;810:120–40.
- Rueth NM, Cromwell KD, Cormier JN. Long-term follow-up for melanoma patients: is there any evidence of a benefit? Surg Oncol Clin N Am 2015;24:359–77.
- Bastiaannet E, Uyl-de Groot CA, Brouwers AH, et al. Cost-effectiveness of adding FDG-PET or CT to the diagnostic work-up of patients with stage III melanoma. Ann Surg 2012;255:771–6.
- Speijers M, Francken A, Hoekstra-Weebers J, et al. Optimal follow-up for melanoma. Expert Rev Dermatol 2010;5:461–78.
- Krug B, Crott R, Lonneux M, et al. Role of PET in the initial staging of cutaneous malignant melanoma: systematic review. Radiology 2008;249:836–44.
- Xing Y, Bronstein Y, Ross MI, et al. Contemporary diagnostic imaging modalities for the staging and surveillance of melanoma patients: a meta-analysis. J Natl Cancer Inst 2011;103:129–42.
- Krug B, Crott R, Roch I, et al. Cost-effectiveness analysis of FDG PET-CT in the management of pulmonary metastases from malignant melanoma. Acta Oncol 2010;49:192–200.
- Rodriguez Rivera AM, Alabbas H, Ramjaun A, et al. Value of positron emission tomography scan in stage III cutaneous melanoma: a systematic review and meta-analysis. Surg Oncol 2014;23:11–6.
- Opfølgningsprogram for modermærkekræft/melanom. Danish Health and Medicine Authority. Internet. 2015. URL: https://sundhedsstyrelsen.dk
- Wasif N, Etzioni D, Haddad D, et al. Staging studies for cutaneous melanoma in the United States: a population-based analysis. Ann Surg Oncol 2015;22:1366–70.
- Stokmo HL, Reitan BC, Johnsen B, et al. Introduction of positron emission tomography into the Western Norwegian Health Region: Regional balance in resource utilization from 2009 to 2014. Clin Physiol Funct Imaging 2015. [Epub ahead of print]. doi: 10.1111/cpf.12335.
- Barsky M, Cherkassky L, Vezeridis M, et al. The role of preoperative positron emission tomography/computed tomography (PET/CT) in patients with high-risk melanoma. J Surg Oncol 2014;109:726–9.
- Baker JJ, Meyers MO, Frank J, et al. Routine restaging PET/CT and detection of initial recurrence in sentinel lymph node positive stage III melanoma. Am J Surg 2014;207:549–54.
- Horn J, Lock-Andersen J, Sjostrand H, et al. Routine use of FDG-PET scans in melanoma patients with positive sentinel node biopsy. Eur J Nucl Med Mol Imaging 2006;33:887–92.
- Bichakjian CK, Halpern AC, Johnson TM, et al. Guidelines of care for the management of primary cutaneous melanoma. American Academy of Dermatology. J Am Acad Dermatol 2011;65:1032–47.
- Balch CM, Gershenwald JE, Soong S, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 2009;27:6199–206.
- Bastiaannet E, Oyen WJ, Meijer S, et al. Impact of [18F]fluorodeoxyglucose positron emission tomography on surgical management of melanoma patients. Br J Surg 2006;93:243–9.
- Petersen H, Holdgaard PC, Madsen PH, et al. FDG PET/CT in cancer: comparison of actual use with literature-based recommendations. Eur J Nucl Med Mol Imaging 2016;43:695–706.
- Schule SC, Eigentler TK, Garbe C, et al. Influence of (18)F-FDG PET/CT on therapy management in patients with stage III/IV malignant melanoma. Eur J Nucl Med Mol Imaging 2016;43:482–8.
- Koskivuo IO, Seppanen MP, Suominen EA, et al. Whole body positron emission tomography in follow-up of high risk melanoma. Acta Oncol 2007;46:685–90.
- Danielsen M, Hojgaard L, Kjaer A, et al. Positron emission tomography in the follow-up of cutaneous malignant melanoma patients: a systematic review. Am J Nucl Med Mol Imaging 2014;4:17–28.
