Abstract
Background: Risk of nodal involvement in patients with squamous cell carcinomas (SCC) of the nasal cavity and maxillary sinus has not been well defined, especially by risk factors beyond local T-stage. Additional criteria defining patients at highest risk, as well as specific nodal levels at highest risk, has been limited in small retrospective series. We describe a population-based assessment of specific nodal involvement in this group.
Material and methods: The Surveillance, Epidemiology and End Results (SEER) database from 2004 to 2010 identified 1283 eligible patients with SCC of the nasal cavity or maxillary sinus. Neck involvement and individual nodal level involvement at presentation were assessed, and comparison made with a contemporaneous cohort of patients with a borderline clinically significant risk of nodal involvement and recurrence.
Results: Among 1283 patients, 182 (14.2%) had nodal involvement at presentation (4–27% by site and local extension). T-stage alone was associated with higher rates of nodal involvement in maxillary sinus SCC, while higher T-stage and size >2 cm were associated with higher rates of nodal involvement in nasal cavity SCC on multivariable analysis. Facial nodes and cervical nodes at levels 1 and 2 have the highest rates of involvement in T4a nasal cavity SCC, whereas nodal levels 1, 2, and/or 3 have the highest rates of involvement in T2 or higher maxillary sinus SCC when compared with a clinical reference standard.
Conclusion: In this population-based study, there are high rates of initial nodal involvement when stratified by local extent determined by T-stage in nasal cavity SCC and maxillary sinus SCC, and independently by size in nasal cavity SCC. Involvement of the facial and nodal levels 1–3 varies depending on site and local extent of tumor involvement. These observations may help guide treatment decision making in the inclusion of and extent of elective nodal treatment fields.
Malignancies of the paranasal sinuses and nasal cavity are rare, comprising <3% of all upper aerodigestive cancers. Among these, squamous cell carcinomas (SCCs) of the nasal cavity and the maxillary sinus are the most common. Incidence and survival in sinonasal cancer has remained relatively stable over the last 30 years, with prognosis dependent on location, histology, and stage [Citation1]. Due to the rarity of nasal cavity and maxillary sinus SCC, treatment recommendations are based on small retrospective series. Standard treatment at the primary site for malignancies of the nasal cavity and maxillary sinus often involves surgical resection followed by either observation or adjuvant radiation with or without chemotherapy [Citation2]. Lymphatic drainage from the maxillary sinus and nasal cavity is heterogeneous, with drainage including connections to the facial/buccal, submandibular, parotid, and parapharyngeal nodal regions [Citation3].
The question of elective neck treatment in the N0 neck for these patients remains to be definitively addressed. The existing literature for nasal cavity SCC and maxillary sinus SCC generally have fewer than 100 patients in each series (). A meta-analysis of literature of 23 retrospective series of nasal cavity SCC, mostly predating modern radiographic and surgical techniques, has found 6% initial nodal involvement and 19% rate of subsequent nodal recurrence. Elective nodal treatment decreases this to a 4% nodal recurrence rate in this meta-analysis [Citation4]. A key disadvantage of existing literature is its dependence on old staging systems predating modern methods of radiographic and surgical staging. In addition, the risk of nodal involvement or recurrence is not stratified by local tumor characteristics but only describes the entire population of nasal cavity SCC. In agreement with the data from the meta-analysis, more recent literature suggests that elective nodal irradiation may be associated with improved regional control [Citation5], but as they combine multiple nasal and paranasal sites and histologies it is impossible to determine rates by primary tumor characteristics, such as T-stage, specifically for nasal cavity SCC [Citation6,Citation7]. The nasal vestibule is a separate site with skin origin and different clinical characteristics from nasal cavity SCC. There is no literature to guide the decision on specific nodal levels to be included in elective radiation or neck dissection fields in nasal cavity SCC.
Table 1. Prior literature on nodal presentation at diagnosis or subsequent nodal recurrence in patients with nasal cavity SCC or maxillary sinus SCC, in those who initially presented with N0 disease.
In maxillary sinus SCC, a study from Loyola of patients who did not receive elective nodal treatment demonstrated a high regional recurrence rate of 11/38 (29%). Of the five patients with T1 or T2, N0 disease at presentation, four (80%) developed regional recurrence [Citation8]. A retrospective series combining the Stanford and UCSF experiences of patients with maxillary sinus SCC, adenocarcinoma, undifferentiated carcinoma, and adenoid cystic carcinoma demonstrates that with an initial clinical N0 neck, the administration of elective neck irradiation decreases nodal relapse from a five-year actuarial rate of 20–0% [Citation9]. As patients with nodal relapse in this study had poor survival, the authors recommend elective ipsilateral upper neck treatment for T3 or higher disease. Of 58 patients with SCC, 16% had nodal involvement at diagnosis, with a 28% overall rate of nodal involvement. All neck recurrences were with T3 or T4 disease, but only five patients had T2 disease. Of 20 patients of all histologies in this study with neck involvement or recurrence, 13 (65%) had ipsilateral level 2 and nine (45%) had ipsilateral level 1, whereas three (15%) had contralateral level 2 neck involvement. An MD Anderson series with maxillary sinus carcinomas of various histologies (36 with SCC) recommends elective nodal treatment for T2-T4 SCC and undifferentiated carcinoma is based on a 33% rate of recurrence in the untreated neck [Citation10]. While not differentiated by histology, 5/14 (36%) of patients with T2 disease had initial or subsequent nodal involvement, with isolated neck recurrence predicting death in 7 of 11 patients. A meta-analysis of maxillary sinus SCC from 12 institutions demonstrates an 11% rate of nodal involvement at presentation, and a 16% rate of delayed neck metastases. An area of disagreement is that some series recommend prophylactic bilateral nodal treatment for T2-T4 maxillary SCC whereas other series recommended elective nodal treatment only for T3-T4 tumors [Citation11]. A report from VU Netherlands of T3-T4 maxillary sinus patients with N0 disease treated with elective ipsilateral neck radiation demonstrated a 4% neck recurrence rate; both of these recurrences occurred in the ipsilateral upper neck [Citation12].
When combining the existing literature, there is a relatively high 25% combined rate of initial (6.6% for nasal cavity and 11.2% for maxillary sinus) and recurrent (18.8% for nasal cavity SCC and 13.1% for maxillary sinus) nodal involvement when elective nodal treatment is not undertaken (). The retrospective series indicate a 5–6-fold decrease in nodal recurrence with elective nodal treatment. It is unclear whether T-stage or other criteria best identifies patients at highest risk for nodal disease meriting elective nodal treatment, and which nodal levels are at highest risk of subclinical involvement. This has left the role of elective nodal treatment at the discretion of the treating physician. For example, MD Anderson includes nasopharyngeal involvement or T4 disease as criteria for elective nodal treatment in nasal cavity SCC although no supporting data is provided [Citation5], while others electively treat the neck in high-grade nasal cavity SCC. For other anatomically distinct but adjacent sites, such as the nasal vestibule, a size cutoff has been used to justify elective nodal treatment. Although elective nodal treatment can consist of either radiotherapy or elective neck dissection, comprehensive neck radiation or neck dissection fields are discouraged due to concerns about patient tolerability. We therefore undertook a population-based analysis to quantify rates of nodal involvement at presentation, with the goal of developing data-driven hypotheses on when and which nodal regions are to be treated electively for these uncommon head and neck cancer subsites.
Material and methods
The study population was extracted from the Surveillance, Epidemiology and End Results (SEER) Program version 8.0.4 (National Cancer Institute, Bethesda, MD), encompassing patients treated from 2004 to 2010. Patients with mucosal SCC of the nasal cavity or maxillary sinus site were included. Staging was according to the American Joint Committee on Cancer (AJCC) 7th edition [Citation13]. There were 956 patients with nasal cavity SCC in the SEER database, with 151 patients excluded due to a prior cancer diagnosis and 72 patients excluded due to lack of primary or nodal staging information, yielding a total of 733 evaluable patients. There were 698 patients with maxillary sinus SCC, with 105 patients excluded due to a prior cancer diagnosis and 43 patients excluded due to lack of primary or nodal staging information, yielding a total of 550 evaluable patients. Size and grade of the primary tumor were recorded in SEER for 425/733 (58%) nasal cavity SCC, whereas size was recorded for 353/550 (64%) of maxillary sinus SCC patients. Nasopharynx involvement was coded in SEER for nasal cavity but not maxillary sinus. Information on nodal involvement was collected as part of the SEER analysis, including involvement of facial, retropharyngeal (RP), each of neck levels 1–5, and parotid as well as contralateral/bilateral nodes.
A limitation of the SEER database is that it limits evaluation of nodal involvement to a period of four months around the patient’s initial diagnosis; it is not possible to obtain a combined rate of initial nodal involvement and subsequent nodal recurrence. In cases in which the rate of initial nodal involvement alone approaches or exceeds 15% in a population-based analysis, the crude incidence alone may support elective nodal treatment [Citation14]. Elective nodal treatment in such a population may lead to an overall survival benefit [Citation15]. However, there is no accepted threshold for elective neck treatment to specific nodal levels.
In order to determine the significance of involvement at presentation of individual nodal levels and to link this to treatment hypotheses, we used comparison to a well established and widely accepted reference standard of patients with head and neck cancer involving another site (T2 glottic larynx) in order to generate clinically meaningful hypotheses regarding the role of elective nodal treatment. This concept and method has been described to make clinically significant hypotheses for elective neck treatment in sinonasal undifferentiated and small cell carcinomas of the paranasal sinuses [Citation16]. The reference standard used for statistical purposes consisted of T2 glottic larynx SCC with hypomobility only and without supraglottic/subglottic invasion in the contemporaneous SEER database. These patients are typically treated to the primary site at the larynx only and, based on the largest series from the University of Florida in Gainesville, are already considered to have a 13% risk of initial neck involvement or subsequent neck recurrence [Citation17]. By contrast, patients with glottic larynx cancer with supraglottic and/or subglottic involvement typically undergo elective treatment to levels 2, 3, and 4 due to a significantly greater risk of nodal involvement at those levels [Citation18]. Owing to the well described natural history of and standard treatment paradigms for T2 glottic laryngeal SCC with cord hypomobility only and without supra/subglottic involvement, any difference in nodal risk for cancers of the nasal cavity and maxillary sinus compared to this subset of T2 glottic larynx patients should meet or exceed the 15% threshold used for elective neck treatment in glottic cancers. In SEER, T2 glottic larynx SCCs with only vocal cord hypomobility demonstrated a 6.16% (31/503) rate of nodal involvement at presentation, whereas T2 glottic larynx SCC with supra/subglottic involvement had a 10.65% (153/1437) rate of nodal involvement at presentation. Of all T2 glottic SCC patients, 93.7% of these patients had either no neck dissection or biopsy of the neck node(s) only. We note that the rate of nodal involvement for T2 glottic larynx SCC at presentation (10.7%) in SEER is lower than the standard 15% threshold for the combination of initial nodal involvement and nodal recurrence, which is expected because SEER contains no data on nodal recurrence after the four-month period.
Using the contemporaneous SEER database, we compared involvement of individual nodal levels 2–4 in nasal cavity and maxillary sinus SCC to the corresponding levels in the reference T2 glottic larynx SCC with vocal cord hypomobility only. Stata v. 13 (Stata Corp., College Station, TX, USA) was utilized for the comparisons. We used logistic regression, χ2, and Fisher exact tests to determine factors at the primary site including T-stage, size, grade and involvement of the nasopharynx that were associated with increased risk of nodal involvement. Due to a non-normal skewed distribution of size of the primary tumor in nasal cavity SCC, a natural logarithmic transformation of size of the primary tumor was used for nasal cavity but not for maxillary sinus SCC. Univariate analysis was performed based on the different criteria at the primary site. For local criteria with p ≤ 0.1, multivariable analysis was performed. For level 1, level 5, parotid, RP, and facial nodes, comparison was made to level 4 involvement of the reference group using Fisher exact test. Statistical significance was ascribed when a two-sided p-value <0.05 was obtained.
Results
A total of 1283 patients with nasal cavity and maxillary sinus SCC were analyzed; 733 patients had nasal cavity SCC (425 with tumor size recorded), whereas 550 patients had maxillary sinus SCC (353 with tumor size recorded). In total 96.6% of patients had no or selective neck dissection, a similar proportion to patients in the reference standard T2 laryngeal cancer group.
In the entire cohort 182 patients (14.2%) had nodal involvement. Bilateral or contralateral neck involvement was present in 42 (3.3%). Skip metastases to secondary echelon nodal levels were negligible (0.08%, 0.16%, and 0.16% for levels 3, 4, and 5, respectively). In unadjusted analysis of nasal cavity SCC T-stage, size, and nasopharyngeal involvement were associated with nodal involvement but grade was not (). On multivariable analysis, overall nodal involvement in nasal cavity SCC was associated with local stage and size but not with grade or nasopharyngeal extension. We found no significant difference in T-stage in patients with and without tumor size information. For patients with maxillary sinus SCC, only T-stage is significant for nodal involvement.
Table 2. T-stage, size, grade, and nasopharyngeal involvement in risk of nodal involvement.
Clinically evident involvement was analyzed separately for each of levels 1–5, parotid, RP, facial nodes, and contralateral neck (). Involvement of each of these levels at presentation was examined for all nasal cavity and maxillary sinus SCC patients (). Patients with nasal cavity SCC had an initial nodal involvement rate of 9.3%. Patients with maxillary sinus SCC had a high rate of initial neck involvement of 20.7%, already exceeding the 15% threshold for combined initial nodal involvement and neck recurrence used for justifying elective nodal treatment. By way of example, this 21% rate of initial involvement for all maxillary sinus SCC was higher than that for a reference standard of T2 glottic larynx patients with supra/subglottic involvement, in whom elective nodal treatment is standard of care (10.7%).
Figure 1. Nodal metastases at presentation: (a) depiction of each of levels 1–5, RP, intraparotid, and facial nodes. In (b) all nasal cavity SCC and (c) all maxillary sinus SCC.
RP: retropharyngeal; SCC: squamous cell carcinoma. Bold values represent p-value ≤0.05.
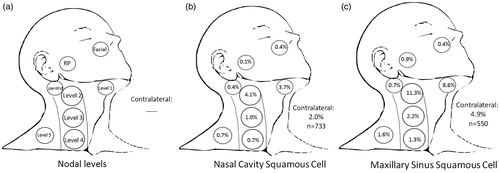
Subgroups at lower and higher risk of nodal involvement were identified. In nasal cavity SCC, T1-T3 patients had lower rates of initial nodal involvement (4–10%, p = NS compared to reference, ), whereas T4a–T4b patients had higher rates (22.2%, p < 0.001). Patients with tumor size between 50th–75th percentile (>20 mm and ≤35 mm, 12.8%, p = 0.024) and tumor size greater than 75th percentile (>35 mm, 10.4%, p = 0.021) have a rate of nodal involvement significantly higher than the reference group. In maxillary sinus SCC, T1 patients have a lower rate of nodal involvement (8.2%, p = NS compared to reference). By contrast, patients with T2 maxillary SCC have a significant risk of nodal involvement that exceeds the combined 15% threshold (18.6%, p = 0.007), which is also the case for patients with T3-4 maxillary sinus SCC (22.3%, p < 0.001).
Table 3. Nodal involvement at presentation by T-stage and size in squamous cell carcinoma of the nasal cavity, and T-stage in maxillary sinus. Reference standard (T2 glottic without supra/subglottic involvement =6.2%). p > 0.05.
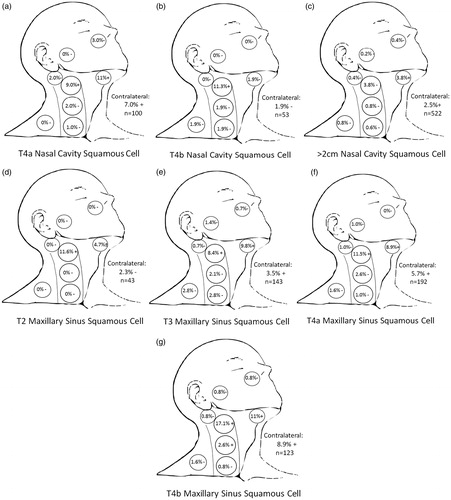
Nodal level involvement in the reference T2 glottic laryngeal cancer without sub/supraglottic and with sub/supraglottic involvement from the contemporaneous SEER database was compared with nodal level involvement in subgroups of nasal cavity () and maxillary sinus SCC (). Patients with T4a nasal cavity SCC, most frequently denoting anterior orbit or skin involvement, had higher and statistically significant rates of level 1–2 and bilateral involvement compared to the reference standard (). For T4a nasal SCC, there was a relatively large absolute incidence (3%) of facial node involvement at presentation, although this was not statistically significant compared to reference due to small numbers. The rate of facial node involvement is noteworthy as this exceeds the 2% rate of initial level 4 involvement in patients with sub/supraglottic spread of glottic cancers, for which standard of care routinely mandates inclusion in elective radiation or neck dissection fields. For patients with T4b nasal cavity SCC, which typically involves the orbital apex, dura or brain, there are higher and statistically significant rates of level 2 involvement only (). For T2 maxillary sinus SCC ipsilateral levels 1 and 2 have a significant rate of nodal involvement, but the contralateral neck does not have a high incidence of involvement (). By contrast, patients with T3-T4b maxillary SCC have higher and statistically significant rates of contralateral neck involvement. This is confined to levels 1–2 for T3 () and T4a (), and additionally a high and statistically significant rate of level 3 involvement for T4b patients (). While there is a relatively higher incidence of RP node involvement at presentation in patients with T3-T4 disease (1.1%) and is highest for patients with T3 disease (1.4%), this does not meet criteria for statistical significance and is lower than the incidence of level 4 involvement in our reference standard (2%).
Figure 2. Nodal levels at clinically significant risk for (a) T4a SCC of the nasal cavity, (b) T4b SCC of the nasal cavity, (c) > 2 cm SCC of the nasal cavity, (d) T2 SCC of the maxillary sinus, (e) T3 SCC of the maxillary sinus, (f) T4a SCC of the maxillary sinus, (g) T4b SCC of the maxillary sinus. (+) denotes p < 0.05 compared to reference standard representing nodal levels to be included in treatment fields. (−) denotes p > 0.05 and therefore nodal levels commonly to be excluded from elective fields.
SCC: squamous cell carcinoma.
Discussion
In this population-based series of patients with nasal cavity and maxillary sinus SCC treated from 2004 to 2010, we find that in nasal cavity SCC there are significantly higher rates of nodal involvement for T4 and primary tumor size ≥2 cm but not for high-grade tumors or those with nasopharyngeal involvement. For maxillary sinus SCC, stage ≥ T2 but not larger size is associated with a significantly and clinically significant higher rate of nodal involvement. These criteria may be used in deciding whether or not to electively treat the clinically negative neck. This is the first study of which we are aware that is composed of patients exclusively diagnosed and treated in an era when modern imaging, such as magnetic resonance imaging (MRI), computed tomography (CT), and positron emission tomography (PET) scans, for staging of the primary and the neck were commonly used allowing high degree of accuracy in assessment of commonly dissected nodal basins in levels 1b–3 as well as in nodal basins that are not commonly dissected including the facial, RP, and parotid levels. This is the first study of which we are aware that examined multiple risk factors at the primary site to predict for nodal involvement, the first study of which we are aware that looked at specific nodal level involvement in nasal cavity SCC, and the largest study to do so in maxillary sinus SCC.
We found that the involved nodal levels, including the facial nodes, each of levels 1–3, and contralateral neck involvement are also dependent on site, T-stage, and/or primary tumor size. In particular, we find that in T4a nasal cavity SCC the facial, levels 1 and 2 and contralateral neck, and in T4b nasal cavity SCC the ipsilateral level 2 are at a higher risk of involvement. Tumors with size >2 cm also have a significant risk for level 1 involvement. Although limited by small numbers (n = 31), nasopharyngeal extension of disease alone is significant by univariate but not multivariable analysis for increased risk of nodal involvement; it is unclear whether SEER differentiates between true mucosal spread of disease to the nasopharynx versus a tumor confined to the mucosa of the nasal cavity but in which a polypoid component enters the nasopharyngeal lumen. For T2 maxillary sinus SCC, the ipsilateral level 1 and 2 are at highest risk of involvement, whereas for T3 maxillary sinus SCC the level 1 and 2, as well as contralateral neck are at highest risk of involvement. Although not statistically significant, our data suggest that T3 maxillary sinus carcinomas may have a higher rate of RP nodal involvement. For T4a maxillary sinus SCC levels 1 and 2 as well as the contralateral neck are at highest risk, whereas for T4b maxillary sinus SCC levels 1, 2, and 3 as well as the contralateral neck are at highest risk of involvement. Our findings suggest a low risk of facial node involvement with all maxillary sinus primaries. Unlike in nasal cavity SCC, we find no correlation between primary tumor size and nodal involvement in maxillary sinus SCC.
Although comprehensive treatment of the bilateral RP and levels 1–5 nodal levels would likely lead to the lowest rates of nodal recurrence, this would be accompanied by significant toxicities. Avoiding comprehensive elective neck radiation or neck dissection to nodal levels with low rates of involvement in patients with N0 disease at presentation may help prevent long-term quality of life issues and allow for lower rates of radiotherapy treatment interruptions. In cases in which adjuvant or definitive radiation therapy is not mandated by characteristics at the primary site, such as in T2 maxillary sinus carcinomas or T1-T2 nasal cavity SCC larger than 2 cm, it may be reasonable for the patient to undergo elective neck dissection, while patients with characteristics mandating adjuvant or definitive radiation therapy (including most T3 and T4 disease) may best be treated with elective neck radiotherapy.
Suggestions for when and which nodal level(s) to treat electively based on initial rates of nodal disease is based on historical precedent. The benefit for elective nodal treatment for SCC of the nasopharynx, oropharynx, oral cavity and larynx was established with pioneering work from MD Anderson in 1972 (Lindberg) [Citation19]. Lindberg’s work tabulates nodal level involvement at initial presentation based on clinical examination only, and without data on nodal recurrence patterns. These rates of involvement at initial presentation were used to rationalize elective nodal treatment, and established that at least levels 2–4 should be included in elective treatment levels for most pharyngeal SCC. However, Lindberg’s study does not provide guidance on an absolute threshold for elective treatment to individual nodal levels. For example, the rate of initial contralateral level 3 nodal involvement for hypopharyngeal carcinomas is only 1.5%, yet this nodal level is always included in elective nodal fields for hypopharyngeal carcinomas and is considered a primary drainage level. As single-institution studies exploring nasal cavity and maxillary sinus SCC provide insufficient numbers to make suggestions regarding which nodal levels should be electively treated based on subpopulations of primary tumor characteristics, such as T-stage, size and grade, we make similar hypotheses based on the larger numbers possible using a population-based study. Similar to the Lindberg study, a series from Memorial Sloan-Kettering utilizing surgical literature was used to generate similar conclusions of nodal risk for different mucosal sites, such as pharyngeal cancers [Citation20]. We have used the SEER database using the same method to reports rates of initial nodal involvement and generate hypotheses on clinically significant nodal levels at risk for sinonasal undifferentiated and small cell carcinomas [Citation16].
Our findings on nodal level involvement may result from how the T classification portion of the AJCC staging system is based on extent and structures involved by the primary. Many T3 maxillary sinus carcinomas involve the posterior maxillary wall, therefore a higher rate of RP nodal involvement might be expected. Conversely, we did not find a high rate of RP nodal involvement in patients with T4b disease (usually denoting significant intracranial extension) with either nasal cavity or maxillary sinus primary. Examination of nasal cavity patients with nasopharyngeal involvement revealed an equivocal increased incidence of RP nodal involvement that was only significant on univariate analysis. In patients with T4a nasal cavity disease there is suggestion of increased facial node involvement; this may be a consequence of the definition of T4a disease including skin involvement. For patients with T4b nasal cavity SCC, there may be a decreased risk of level 1 involvement as T4b disease is defined as involvement of more posterior and superior structures, such as the skull base, orbital apex or nasopharynx. There is a clinically higher rate of level 1 involvement in T4b maxillary sinus cancers even though the definition for T4b maxillary sinus cancer is similar to that for nasal cavity. Although some institutions have decided to base treatment of the neck on factors, such as T-stage or grade, we find no association of grade but an association with size >2 cm with risk of nodal involvement for nasal cavity and no association with size for maxillary sinus.
One weakness of this study is that some patients underwent neck dissection, which could confound determination of nodal involvement due to its greater sensitivity compared to imaging studies for nodal staging. The similar rates of neck dissection in patients with the reference standard T2 glottic cancers may minimize the influence of this confounder. Although SEER gives information on whether there is ipsilateral or contralateral involvement, it does not otherwise give information on laterality of the involved nodal level. Even though it is unknown which imaging modality was used in each patient and different imaging modalities, such as PET, CT, MRI and ultrasound may have varying rates of sensitivity and specificity, each is considered to be highly sensitive and accurate in detection of nodal disease [Citation21]. A further disadvantage of this work is that observational studies, such as SEER, suffer from bias from unmeasured confounding as a limitation, including information on how patients were staged. We attempted to mitigate this confounding by using the reference group. Additionally, akin to the experience from the 1960s and 1970s noted above for Lindberg’s study of head and neck mucosal SCC, a significant potential flaw in our methodology is the assumption that recurrence risks are proportional to rates of initial nodal involvement and our further assumption that the ratio of initial nodal presentation to subsequent nodal recurrence is similar between our nasal and paranasal population and that of the T2 glottic reference group.
Similar studies with SEER have been performed in other sites. Data in SEER is considered to be 98% accurate [Citation22], and subject to a continuous quality improvement process [Citation23]. Primary site and nodal data from SEER has been used to correlate nodal involvement and nodal count with survival in gastric cancer [Citation24], and to find predictive factors for nodal metastasis in major and minor salivary gland cancers [Citation25] based on primary site characteristics. Although our study was population based and is the largest study of which we are aware, many of the subcategories of site and stage nevertheless have small patient numbers. Therefore, fully adjusted statistical models were not possible and specific recommendations, such as treatment of facial nodes, must be made based solely on raw incidence rates. Although we have generated hypotheses concerning whether and to which nodal levels elective treatment of the neck may be performed in patients with clinical N0 disease, patients with N + clinical nodal involvement at presentation would likely warrant dissection or nodal irradiation to a comprehensive neck field.
As population-based studies, such as SEER, have limitations secondary to the lack of patterns of failure data, only hypotheses regarding the role and elective fields for treatment or observation of the clinically uninvolved neck can be generated. Our findings from SEER demonstrate high rates of initial nodal involvement especially for specific subgroups, generating a hypothesis that subgroups of nasal cavity SCC based on higher T-stage and larger size and subgroups of maxillary sinus SCC based on higher T-stage have a high risk of clinical or subclinical nodal involvement. We find particular nodal levels at highest risk may be dependent on characteristics of disease at the involved sinonasal subsite. However, we would caution that both this work and associated review of the literature is only suggestive that elective neck treatment is warranted in patients with nasal cavity or maxillary sinus SCC, and do not provide a definitive level of proof about the specific nodal levels at clinically significant risk of involvement.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Turner JH, Reh DD. Incidence and survival in patients with sinonasal cancer: a historical analysis of population-based data. Head Neck 2012;34:877–85.
- Katz TS, Mendenhall WM, Morris CG, et al. Malignant tumors of the nasal cavity and paranasal sinuses. Head Neck 2002;24:821–9.
- Lang J. Clinical anatomy of the nose, nasal cavity and paranasal sinuses. Stuttgart: Thieme; 1989;
- Scurry WC, Jr., Goldenberg D, Chee MY, et al. Regional recurrence of squamous cell carcinoma of the nasal cavity: a systematic review and meta-analysis. Arch Otolaryngol Head Neck Surg 2007;133:796–800.
- Allen MW, Schwartz DL, Rana V, et al. Long-term radiotherapy outcomes for nasal cavity and septal cancers. Int J Radiat Oncol Biol Phys 2008;71:401–6.
- Hoppe BS, Stegman LD, Zelefsky MJ, et al. Treatment of nasal cavity and paranasal sinus cancer with modern radiotherapy techniques in the postoperative setting—the MSKCC experience. Int J Radiat Oncol Biol Phys 2007;67:691–702.
- Daly ME, Chen AM, Bucci MK, et al. Intensity-modulated radiation therapy for malignancies of the nasal cavity and paranasal sinuses. Int J Radiat Oncol Biol Phys 2007;67:151–7.
- Paulino AC, Fisher SG, Marks JE. Is prophylactic neck irradiation indicated in patients with squamous cell carcinoma of the maxillary sinus? Int J Radiat Oncol Biol Phys 1997;39:283–9.
- Le QT, Fu KK, Kaplan M, et al. Treatment of maxillary sinus carcinoma: a comparison of the 1997 and 1977 american joint committee on cancer staging systems. Cancer 1999;86:1700–11.
- Jiang GL, Ang KK, Peters LJ, et al. Maxillary sinus carcinomas: natural history and results of postoperative radiotherapy. Radiother Oncol 1991;21:193–200.
- Rinaldo A, Ferlito A, Shaha AR, et al. Is elective neck treatment indicated in patients with squamous cell carcinoma of the maxillary sinus? Acta Otolaryngol 2002;122:443–7.
- Jeremic B, Shibamoto Y, Milicic B, et al. Elective ipsilateral neck irradiation of patients with locally advanced maxillary sinus carcinoma. Cancer 2000;88:2246–51.
- Edge SB, Compton CC. The american joint committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471–4.
- Pitman KT. Rationale for elective neck dissection. Am J Otolaryngol 2000;21:31–7.
- D'cruz AK, Vaish R, Kapre N, et al. Elective versus therapeutic neck dissection in node-negative oral cancer. N Engl J Med 2015;373:521–9.
- Ahn PH, Mitra N, Alonso-Basanta M, et al. Nodal metastasis and elective nodal level treatment in sinonasal small-cell and sinonasal undifferentiated carcinoma: a surveillance, epidemiology and end results analysis. Br J Radiol 2016;89:20150488.
- Mendenhall WM, Amdur RJ, Morris CG, et al. T1-T2N0 squamous cell carcinoma of the glottic larynx treated with radiation therapy. J Clin Oncol 2001;19:4029–36.
- Gregoire V, Coche E, Cosnard G, et al. Selection and delineation of lymph node target volumes in head and neck conformal radiotherapy. proposal for standardizing terminology and procedure based on the surgical experience. Radiother Oncol 2000;56:135–50.
- Lindberg R. Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer 1972;29:1446–9.
- Shah JP. Patterns of cervical lymph node metastasis from squamous carcinomas of the upper aerodigestive tract. Am J Surg 1990;160:405–9.
- Adams S, Baum RP, Stuckensen T, et al. Prospective comparison of 18F-FDG PET with conventional imaging modalities (CT, MRI, US) in lymph node staging of head and neck cancer. Eur J Nucl Med 1998;25:1255–60.
- Harlan LC, Hankey BF. The surveillance, epidemiology, and end-results program database as a resource for conducting descriptive epidemiologic and clinical studies. J Clin Oncol 2003;21:2232–3.
- Available from http://seer.cancer.gov/qi/tools/reliability.html
- Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. J Clin Oncol 2005;23:711424.
- Lloyd S, Yu JB, Ross DA, et al. A prognostic index for predicting lymph node metastasis in minor salivary gland cancer. Int J Radiat Oncol Biol Phys 2010;76:169–75.
