Abstract
Background: Although the spectrum of systemic treatment for metastatic colorectal cancer (mCRC) has widened, there is a paucity of evidence for the feasibility and optimal use of these systemic agents in elderly patients. The present study provides real world data on the age-related systemic treatment and survival of CRC patients with non-resectable metachronous metastases.
Methods: All consecutive patients with non-resectable metastases from primary resected CRC were extracted from the Eindhoven area of the Netherlands Cancer Registry (NCR). Patients receiving palliative systemic therapy were enrolled (n = 385). Systemic treatment and survival were analyzed according to age at diagnosis of metastases.
Results: Patients aged ≥75 years more often received first-line single-agent chemotherapy than their younger counterparts (63% vs. 32%, p < .0001). First-line single-agent chemotherapy was often prescribed without additional targeted therapy (78%). Advanced age (≥75 years) was associated with a lower probability of receiving all active cytotoxic agents compared to patients aged <60 years at time of diagnosis of metastases (odds ratio (OR) 0.2, 95% CI 0.10–0.77). In a multivariable Cox regression analysis with adjustment for age and other relevant prognostic factors, the total number of received systemic agents was the only predictor of death (hazard ratio (HR) 0.7, 95% CI 0.61–0.81).
Conclusion: The beneficial effect of treatment with all active systemic agents on survival (simultaneously or sequentially prescribed) should be taken into account when considering systemic therapy in patients with mCRC. In light of our results, future studies are warranted to clarify the role of potential targeted therapy in elderly mCRC patients, who are often not candidates for combination chemotherapy and treatment with all active cytotoxic agents.
Colorectal cancer (CRC) is the second most lethal cancer in the Netherlands. Most cancer-related deaths result from the progressive growth of metastases, which are present at time of diagnosis in approximately 20% of the patients [Citation1–3] or occur during the course of disease in another 14–34% of the patients [Citation4–8].
Since the late 1990s, the spectrum of systemic treatment in metastatic CRC (mCRC) has widened. Various systemic regimens combining fluoropyrimidines, oxaliplatin and irinotecan have become available, and more recently different monoclonal antibodies were introduced including bevacizumab, cetuximab and panitumumab.
The use of targeted therapy in addition to the available cytotoxic agents has been positively associated with survival [Citation9,Citation10]. The feasibility and optimal sequence of the administration of these systemic agents in elderly patients, however, is unclear. With the traditional under-representation of elderly patients in clinical trials [Citation11], randomized data are scarce. High quality real-life studies are needed as the increasing proportion of elderly mCRC patients poses significant challenges to cancer specialists. The aim of the current study was to provide insight into the impact of age on the systemic treatment and survival of patients with unresectable metachronous metastases from CRC outside the setting of a randomized clinical trial.
Methods
Data collection
Data from the population-based Netherlands Cancer Registry (NCR), more specifically from the Eindhoven area, were used. This registry records data on all patients with newly diagnosed cancer in the southern part of the Netherlands, an area with approximately 2.4 million inhabitants (∼15% of the Dutch population), six pathology departments, 10 hospitals and two radiotherapy institutions. Information on patient and tumor characteristics are collected from medical records by specially trained registry staff after notification by pathologists and medical registration offices, resulting in high quality of the data. In the NCR, primary tumors are classified according to the TNM classification of Malignant Tumors by the International Union Against Cancer (UICC), seventh edition [Citation12]. Anatomical site of the tumor is registered according to the International Classification of Diseases for Oncology (ICD-O). A slightly modified version of the Charlson comorbidity index was used to register comorbidities.
For the present study, additional data were retrospectively collected between 2010 and 2011 on metachronous metastases for patients diagnosed between 2003 and 2008 with non-mCRC (stage I–III). Hospitals were asked to participate in the study by giving permission to use their data from the NCR and by giving permission for the retrospective registration of additional data. Metachronous metastases were defined as distant metastases of primary CRC in other organs, diagnosed at least three months after CRC diagnosis. Median time from primary diagnosis to data collection was 5.3 years (1.5–8.8 years). The additional data collection encompassed detailed information on systemic therapy, both chemotherapy and targeted therapy.
All consecutive patients with metachronous metastases from primary resected stage I–III CRC (C18.0–C18.9, C190, C209) were selected (n = 1007). Patients undergoing surgery for metastases (n = 261) or only supportive care (n = 361) were excluded for the present study, resulting in a study population of patients treated with palliative systemic therapy (n = 385). Patients were divided into categories according to their age at time of metachronous metastases diagnosis (<60 years, 60–75 years, ≥75 years) and palliative systemic treatment was assessed according to the number of received systemic agents and systemic treatment lines.
Statistical analyses
Descriptive statistics were used to provide an overview on patient and tumor characteristics of the total study population (n = 385). First-line systemic regimens were categorized according to the number of prescribed cytotoxic agents (single-agent chemotherapy, combination-chemotherapy) and the additional prescription of targeted therapy. Variation in the use of these systemic regimens between age categories was assessed using a χ2-test. Duration of first-line treatment was calculated and presented as median duration in months. Differences in first-line duration between age categories were assessed and tested using a Wilcoxon rank sum test. Subsequently, proportions of patients receiving second-line systemic therapy were calculated and a multivariable logistic regression analysis was applied to investigate the independent influence of age on the receipt of second-line therapy. Adjustments were made for relevant patient and tumor characteristics: gender, comorbidity and socioeconomic status at time of CRC diagnosis, primary tumor localization, adjuvant chemotherapy, time to metastases, period of diagnosis of metastases, follow-up time since metastases diagnosis, the number of affected organs and the prescribed first-line regimen. This model was also applied to investigate the influence of age on the odds of exposure to all three active cytotoxic agents.
Overall survival (OS) time was defined as the time from diagnosis of the first metachronous metastatic site to death or lost to follow-up. Patients still alive at the end of follow-up (1 February 2016) and those who emigrated were censored. Crude survival estimates were calculated for both the total study population and according to age with the Kaplan-Meier method; crude survival rates were presented up to 48 months. A log-rank test was carried out to evaluate differences between survival curves. Median survival (MS) was presented in months and corresponding 95% confidence intervals (CIs). Multivariable Cox regression analyses were used to identify independent prognostic factors. Adjustments were made for clinically relevant variables that were applied in the multivariable logistic regression analysis, also including the total number of received systemic agents. p-Values below .05 were considered statistically significant. SAS/STAT® statistical software (SAS system 9.4, SAS Institute, Cary, NC) was used for all analyses.
Results
In total, 385 patients received palliative systemic therapy for the treatment of metachronous metastases from primary resected CRC. Mean age at time of metachronous metastases diagnosis was 67.5 years [standard deviation (SD) 10, range 26–90 years]. An overview of patient and tumor characteristics is shown in .
Table 1. Patient and tumor characteristics of the total study population of patients with metachronous metastases from primary resected stage I–III CRC treated with palliative systemic therapy (n = 385).
First-line systemic therapy
provides an overview on the palliative systemic treatment of metachronous metastases from primary resected CRC. Of the total number of 385 patients, 60% received first-line combination chemotherapy (of which 94% oxaliplatin-based) and 40% received single-agent chemotherapy (of which 82% fluoropyrimidines). Targeted agents (mostly bevacizumab) were prescribed in 174 patients (45%), primarily in addition to combination chemotherapy. Significant differences in first-line systemic regimens were observed between age categories (); elderly patients (≥75 years) more often received single-agent chemotherapy than their younger counterparts (63% ≥75 years vs. 27% <60 years).
Figure 1. First-line chemotherapeutic regimens according to age at time of diagnosis of metachronous metastases (n = 385).
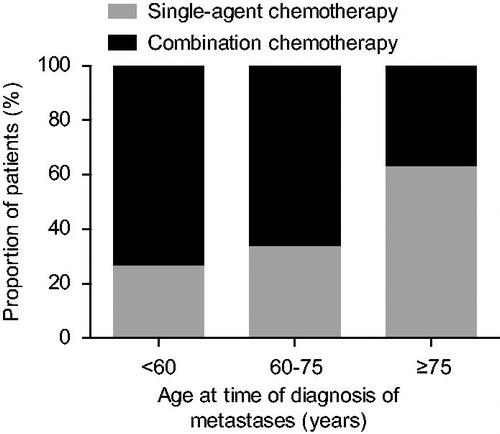
Table 2. Palliative systemic treatment of metachronous metastases from primary resected CRC, according to age at diagnosis of metastatic disease (n = 385).
Median duration of first-line treatment was 3.6 months [interquartile range (IQR) 1.3–8.4]. Significant differences in first-line treatment time were observed between age categories, with respectively 4.1 months (IQR 2.06–11.72) in patients <60 years, 3.6 months (IQR 1.57–8.37) in patients aged 60–75 years and 3.4 months (IQR 0.68–7.59) in patients aged ≥75 years.
Second and further lines of systemic treatment
Less than half of the patients (40%) received second-line therapy (n = 154, ). With increasing age, the proportion of patients receiving secondary treatment decreased, from 55% in patients <60 years to 26% in patients aged ≥75 years (p < .0001, ). This was confirmed in a multivariable analysis in which patients aged ≥75 years at time of metachronous metastases diagnosis were less likely to receive second-line treatment than patients aged <60 years (odds ratio (OR) 0.3, 95% CI 0.16–0.80) (see ).
Figure 2. Proportion of patients receiving second and further lines of systemic treatment (a), according to age at time of diagnosis of metachronous metastases (b) (n = 385).
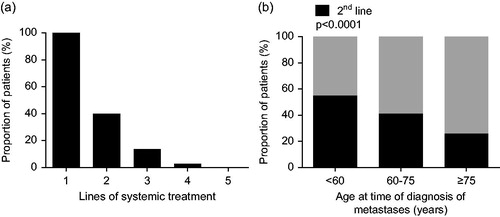
Table 3. Predictors of treatment with second-line treatment and exposure to all three available cytotoxic agents, adjusted for all factors listed (n = 385).
Total number of received systemic agents during treatment course
–cytotoxic agents
Fluoropyrimidines were prescribed at any time during treatment course in 93% of the patients (mostly capecitabine), whereas oxaliplatin and irinotecan were prescribed in respectively 61% and 37%. A minority of the patients (22%) were exposed to all three cytotoxic agents (). Advanced age (≥75 years) was associated with a lower probability to receive all three active cytotoxic agents compared to patients aged <60 years at time of metastases diagnosis (OR 0.2, 95% CI 0.10–0.77, ). Patients receiving first-line combination chemotherapy were more likely to receive all three cytotoxic agents than patients treated with first-line single-agent chemotherapy (OR 7.1, 95% CI 3.16–16.11).
–targeted agents
In total, 56% of the patients received additional targeted therapy during their course of disease (n = 216). Over time, the use of targeted therapy increased from 30% in 2003–2005 to 64% in 2009–2011. This trend was observed regardless of age, although in elderly patients (≥75 years) proportions increased primarily since 2009 whereas the increase in younger patients was observed already since 2005. Overall, proportions were significantly lower in patients aged ≥75 years (37%).
Bevacizumab was the most frequently prescribed targeted agent (n = 207), which was added primarily to first-line systemic therapy (81%). Epidermal growth factor (EGFR) inhibitors (cetuximab/panitumumab) were administered to 35 patients (9%), mainly in addition to second-line treatment (n = 23).
Survival and predictors of death
Median OS of the total study population was 16.6 months (95% CI 14.42–19.19). Significant differences in OS time were observed between age categories, with respectively 14.2 months (95% CI 11.33–16.69) in patients ≥75 years and 20.3 months (95% CI 13.96–22.60) in patients <60 years (p < .01). After adjustment for relevant patient and tumor characteristics and treatment variables (first-line systemic therapy, number of exposed systemic agents) advanced age (≥75 years) was no longer significantly associated with OS (hazard ratio (HR) 1.3, 95% CI 0.90–1.86, p = .16). Although significant in univariate analysis (HR 0.7, 95% CI 0.57–0.86, p < .01), first-line combination chemotherapy also did not achieve significance in multivariate analysis (HR 1.2, 95% CI 0.86–1.56, p = .33), but the number of exposed systemic agents remained significantly associated with OS (HR 0.7, 95% CI 0.61–0.81, p < .0001).
Discussion
In the present population-based study we provided insight into the age-related systemic treatment and survival of patients with unresectable metachronous metastases from primary resected CRC.
We demonstrated that in everyday clinical practice only 26% of the elderly patients started second-line treatment. As most elderly patients received first-line single-agent chemotherapy without targeted therapy, elderly patients were less likely to receive all active systemic agents during their course of treatment, which was associated negatively with survival.
Overall, more than half (60%) of the mCRC patients received first-line combination chemotherapy with or without a targeted agent. As first-line treatment, combination chemotherapy has been associated with prolonged progression-free survival (PFS) and OS compared with single-agent chemotherapy [Citation13–16]. Nevertheless, only a minority of the elderly patients (≥75 years) received combination chemotherapy (37%), probably due to concerns on tolerability and toxicity. For a subgroup of patients with indolent disease, irrespective of age, there is no indication for combination chemotherapy. In the FOCUS2 trial investigating chemotherapy options in frail and elderly patients with advanced CRC, patients were randomly assigned to either intravenous fluorouracil with levofolinate, capecitabine, oxaliplatin and fluorouracil with levofolinate or oxaliplatin and capecitabine. Treatment was started at 80% of the standard dose as full dose regimens are often considered unsuitable in elderly and frail patients. The addition of oxaliplatin did not improve PFS [Citation17]. Besides, the French FFCD2001-02 trial failed to demonstrate improved OS rates with irinotecan combination-chemotherapy versus single-agent fluorouracil with levofolinate although even greater toxicity rates were reported with irinotecan combination chemotherapy [Citation18].
Regardless of the sequence of administration, exposure to all active cytotoxic agents during treatment has been associated with prolonged survival [Citation19]. In view of this observation, it has been suggested that the sequential use of active single agents might be preferable to initial combination chemotherapy as this could conceivably reduce overall toxicity. Three European trials directly addressed this issue. In the FOCUS and FFCD 2000-05 trial, initial monotherapy followed by combination chemotherapy was non-inferior to initial combination therapy [Citation20,Citation21]. These results were endorsed by the CAIRO trial in which the sequential treatment strategy (first-line capecitabine, second-line irinotecan, third-line CAPOX) provided a similar benefit to initial combination treatment (first-line CAPIRI, second-line [Citation22] CAPOX) [Citation23].
The sequential treatment strategy, however, has several limitations. At first, it should not be initiated patients with potentially resectable metastases or severe cancer-related symptoms in whom the primary goal is downsizing of the tumor, as response rates are superior with combination chemotherapy [Citation13,Citation15]. Besides, sequential treatment implies that patients are still fit for second and further lines of treatment after progressing, which might not be the case in patients with an aggressive disease or a poor performance status. According to the study by Grothey et al., with data from seven phase III trials, 50–80% of the patients received second-line treatment after failure of first-line treatment [Citation19]. In the present population-based study, higher dropouts rates were observed in everyday clinical practice (60% after first-line) due to the impact of a relatively large number of elderly patients. Only 26% of the elderly patients (≥75 years) received second-line therapy. This percentage is in line with the study by Sorbye et al., in which a poor performance status at start of first-line chemotherapy was identified as a poor predictor for administration of second-line treatment [Citation24].
In our study, only 22% of the mCRC patients were exposed to all three cytotoxic drugs during their course of treatment. The likelihood of receiving all active cytotoxic agents was significantly lower with the use of first-line single-agent chemotherapy (5%) than with initial combination therapy (33%). These results are in line with data from the FOCUS and CAIRO trial, although proportions of patients receiving all cytotoxic agents in these two trials were higher. With the sequential treatment, 19% of the patients in the FOCUS trial and 36% of the patients in the CAIRO trial received all cytotoxic agents, whereas proportions were respectively 33% and 55% with initial combination treatment [Citation21,Citation23]. The dismal proportions as observed in our study probably arose from the relatively large proportion of elderly patients in daily based practice. In the present study, advanced age (≥75 years) was independently associated with a lower probability to access all three active cytotoxic drugs compared to patients aged <60 years.
During the current study period, elderly patients (≥75 years) were not only less likely to receive all active cytotoxic agents during their course of treatment, but also less frequently received targeted therapy. Initially, evidence on the use of bevacizumab – the first available and registered targeted agent in The Netherlands – was derived from a trial in which a currently outdated chemotherapy regimen (IFL) was used and elderly patients were under-represented [Citation9]. Nowadays, several studies have suggested that bevacizumab is both safe and effective in combination with multiple chemotherapy backbone regimens [Citation25], also in elderly patients [Citation26–28] and that age itself should no longer be regarded an absolute contraindication. Probably as a result, bevacizumab was prescribed increasingly over time [Citation22], also in elderly mCRC patients. Evidence on the use of other targeted agents such as anti-EGFR therapies (cetuximab, panitumumab) in elderly KRAS-wild type mCRC patients, however, remains scarce and less clear [Citation29]. Recently, it has been suggested that single-agent panitumumab may be a well tolerated and active therapeutic option for frail elderly patients with wild-type RAS tumors [Citation30]. More studies are needed to clarify the role of anti-EGFR therapies in the population of elderly mCRC patients, especially as targeted agents may sometimes be the only therapeutic option for frail elderly patients who are unable to tolerate chemotherapy.
Several phase III trials [Citation13–15,Citation31,Citation32] and retrospective cohort studies [Citation22,Citation33] have demonstrated survival rates exceeding 21.5 months in mCRC patients treated with modern systemic regimens, which seems in line with the median OS of 20.2 months in patients <60 years as observed in our study. Inferior results, however, were observed in elderly mCRC patients (≥75 years), with a median OS of 14 months. These results, along with results from a prior Nordic population-based registry [Citation34], raise concerns over our ability to improve treatment options for elderly mCRC patients. In a multivariate analysis with adjustments for available prognostic factors, we found that only the number of exposed systemic agents was associated with OS, which suggests the need of a strategy to make all active agents available to patients with mCRC. Of course, these results need to be interpreted with caution due to the invariable presence of selection bias in this non-randomized study (patients who receive all drugs must live longer, since they need to be in shape for this), which cannot be fully out ruled in a multivariate analysis. Nevertheless, our results indicate that in elderly patients, initial treatment with the highest potential of improving both survival and maintaining quality of life is needed as most of these patients are not candidates for second-line treatment. Further studies are warranted to further define the role of targeted therapy in elderly mCRC patients who often are not candidates for intensive chemotherapy.
To the best of our knowledge, this is the first population-based study describing the whole spectrum of systemic treatment and survival of a long-term series of consecutive CRC patients with non-resectable metachronous metastases. The non-randomized nature of this study also presents a potential risk of (selection) bias. Reasons (not) to prescribe specific systemic regimens were not available. Besides, relevant patient characteristics such as comorbidity and performance score were registered only at initial CRC diagnosis. Moreover, performance score was often not noted in patient charts (missing in >50%) and as a result, these data were not useful. Data on RAS/BRAF mutation status were also not present. Despite these limitations, this large population-based study presents real world data which are of need in today’s developing cancer care.
Conclusion
In daily practice, most elderly patients with non-resectable metachronous metastases from primary resected CRC receive first-line single-agent chemotherapy without a targeted therapy. Only a minority of the elderly mCRC patients receive a second line of treatment. As a consequence, very few elderly patients received all active systemic agents during their course of treatment, shown to be the only independent predictor of death. Future studies are needed to clarify the role of targeted therapy in elderly mCRC patients, who are often not candidates for combination chemotherapy.
Acknowledgments
The authors thank the registration team of the Netherlands Cancer Registry for their dedicated data collection. Furthermore, we would like to thank all the contributing hospitals: Amphia Hospital, Breda; Catharina Hospital, Eindhoven; Elkerliek Hospital, Helmond and Deurne; Hospital Bernhoven, Bernhoven; Jeroen Bosch Hospital, 's Hertogenbosch; Máxima Medical Center, Eindhoven and Veldhoven; St. Anna Hospital, Geldrop; Elisabeth-TweeSteden Hospital, Tilburg and Waalwijk; VieCuri Hospital, Venlo and Venray.
Disclosure statement
Both funders did not have any involvement in the study design, the collection, analysis and interpretation of data; in writing of the manuscript; and in the decision to submit the manuscript for publication.
The authors declare that they have no conflict of interest.
References
- Surveillance, Epidemiology, and End Results Program (SEER). Available from: http://seer.cancer.gov/statfacts [last accessed 2016].
- Lemmens V, van Steenbergen L, Janssen-Heijnen M, et al. Trends in colorectal cancer in the south of the Netherlands 1975-2007: rectal cancer survival levels with colon cancer survival. Acta Oncol 2010;49:784–96.
- Meulenbeld HJ, van Steenbergen LN, Janssen-Heijnen ML, et al. Significant improvement in survival of patients presenting with metastatic colon cancer in the south of The Netherlands from 1990 to 2004. Ann Oncol 2008;19:1600–4.
- Galandiuk S, Wieand HS, Moertel CG, et al. Patterns of recurrence after curative resection of carcinoma of the colon and rectum. Surg Gynecol Obstet 1992;174:27–32.
- Manfredi S, Bouvier AM, Lepage C, et al. Incidence and patterns of recurrence after resection for cure of colonic cancer in a well defined population. Br J Surg 2006;93:1115–22.
- Obrand DI, Gordon PH. Incidence and patterns of recurrence following curative resection for colorectal carcinoma. Dis Colon Rectum 1997;40:15–24.
- Guyot F, Faivre J, Manfredi S, et al. Time trends in the treatment and survival of recurrences from colorectal cancer. Ann Oncol 2005;16:756–61.
- van Gestel YR, de Hingh IH, van Herk-Sukel MP, et al. Patterns of metachronous metastases after curative treatment of colorectal cancer. Cancer Epidemiol 2014;38:448–54.
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335–42.
- Van Cutsem E, Lenz HJ, Kohne CH, et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol 2015;33:692–700.
- Jennens RR, Giles GG, Fox RM. Increasing underrepresentation of elderly patients with advanced colorectal or non-small-cell lung cancer in chemotherapy trials. Intern Med J 2006;36:216–20.
- UICC. TNM classification of malignant tumors. 7th ed. Wiley-Blackwell: New York, 2009.
- Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomized trial. Lancet 2000;355:1041–7.
- Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med. Irinotecan Study Group. 2000;343:905–14.
- de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 2000;18:2938–47.
- Giacchetti S, Perpoint B, Zidani R, et al. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol 2000;18:136–47.
- Seymour MT, Thompson LC, Wasan HS, et al. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): an open-label, randomized factorial trial. Lancet 2011;377:1749–59.
- Aparicio T, Lavau-Denes S, Phelip JM, et al. Randomized phase III trial in elderly patients comparing LV5FU2 with or without irinotecan for first-line treatment of metastatic colorectal cancer (FFCD 2001-02)dagger. Ann Oncol 2016;27:121–7.
- Grothey A, Sargent D, Goldberg RM, et al. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol 2004;22:1209–14.
- Ducreux M, Malka D, Mendiboure J, et al. Sequential versus combination chemotherapy for the treatment of advanced colorectal cancer (FFCD 2000-05): an open-label, randomized, phase 3 trial. Lancet Oncol 2011;12:1032–44.
- Seymour MT, Maughan TS, Ledermann JA, et al. Different strategies of sequential and combination chemotherapy for patients with poor prognosis advanced colorectal cancer (MRC FOCUS): a randomized controlled trial. Lancet 2007;370:143–52.
- Tomita Y, Karapetis CS, Ullah S, et al. Survival improvements associated with access to biological agents: Results from the South Australian (SA) metastatic colorectal cancer (mCRC) registry. Acta Oncol 2016;55:480–5.
- Koopman M, Antonini NF, Douma J, et al. Sequential versus combination chemotherapy with capecitabine, irinotecan, and oxaliplatin in advanced colorectal cancer (CAIRO): a phase III randomized controlled trial. Lancet 2007;370:135–42.
- Sorbye H, Berglund A, Tveit KM, et al. Secondary treatment and predictive factors for second-line chemotherapy after first-line oxaliplatin-based therapy in metastatic colorectal cancer. Acta Oncol 2007;46:982–8.
- Wagner AD, Arnold D, Grothey AA, et al. Anti-angiogenic therapies for metastatic colorectal cancer. Cochrane Database Syst Rev 2009;3:CD005392.
- Kabbinavar FF, Hurwitz HI, Yi J, et al. Addition of bevacizumab to fluorouracil-based first-line treatment of metastatic colorectal cancer: pooled analysis of cohorts of older patients from two randomized clinical trials. J Clin Oncol 2009;27:199–205.
- Kozloff M, Yood MU, Berlin J, et al. Clinical outcomes associated with bevacizumab-containing treatment of metastatic colorectal cancer: the BRiTE observational cohort study. Oncologist 2009;14:862–70.
- Cunningham D, Lang I, Marcuello E, et al. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): an open-label, randomized phase 3 trial. Lancet Oncol 2013;14:1077–85.
- Rosati G, Aprile G, Cardellino GG, et al. A review and assessment of currently available data of the EGFR antibodies in elderly patients with metastatic colorectal cancer. J Geriatr Oncol 2016;7:134–41.
- Sastre J, Massuti B, Pulido G, et al. First-line single-agent panitumumab in frail elderly patients with wild-type KRAS metastatic colorectal cancer and poor prognostic factors: a phase II study of the Spanish Cooperative Group for the Treatment of Digestive Tumors. Eur J Cancer 2015;51:1371–80.
- Tournigand C, Andre T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 2004;22:229–37.
- Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 2004;22:23–30.
- Hammerman A, Greenberg-Dotan S, Battat E, et al. The 'real-life' impact of adding bevacizumab to first-line therapy in metastatic colorectal cancer patients: a large Israeli retrospective cohort study. Acta Oncol 2015;54:164–70.
- Sorbye H, Cvancarova M, Qvortrup C, et al. Age-dependent improvement in median and long-term survival in unselected population-based Nordic registries of patients with synchronous metastatic colorectal cancer. Ann Oncol 2013;24:2354–60.
