To the Editor,
The most common complication from the treatment of prostate cancer is erectile dysfunction (ED) [Citation1]. Physician-reported data suggests this problem occurs in at least one third to one half of men, but patient-reported outcomes suggest the incidence may be significantly higher [Citation2–4].
Currently, medical management using the phophodiesterase-5 (PDE5) inhibitors tends to be effective in the majority of patients [Citation5]. Unfortunately, the out-of-pocket costs for these medicines prove unaffordable to most men. Thus, there is a need for less costly approaches to the prevention and treatment of ED in men with prostate cancer.
The 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors (statins) have been studied as a potential treatment for ED [Citation6], although not in the setting of radiation therapy. We recently reported on a phase II trial of lovastatin as a potential means to prevent rectal injury in men receiving radiation therapy for prostate cancer [Citation7]. A secondary endpoint of this trial was the impact of lovastatin on ED, using patient-reported outcomes as the measure of efficacy. Herein, we report the results of this analysis.
Material and methods
The study was approved by the institutional review boards of the participating institutions and in accordance with an assurance filed with and approved by the US Department of Health and Human Services. All patients signed written informed consent prior to enrollment. The trial was registered with Clinicaltrials.gov (registration number: NCT00580970).
Details of eligibility and methodology have been reported elsewhere [Citation7] and will be summarized briefly here. Eligible patients were those with adenocarcinoma of the prostate to be treated with radiation therapy with curative intent. The planned total dose to the planning target volume had to be at least 60 Gy such that a portion of the rectum received a minimum exposure of at least 60 Gy.
Baseline genitourinary, gastrointestinal and sexual function was assessed using both patient-reported data [EPIC (Expanded Prostate Cancer Index Composite: urinary, bowel, sexual, hormonal modules and overall satisfaction), IIEF (International Index of Erectile Function)] and physician-reported data [CTCAE v3 (Common Terminology Criteria for Adverse Events Version 3: genitourinary and gastrointestinal adverse events)].
The doses to target volumes and normal tissues were determined using established departmental guidelines for both external beam radiation therapy (EBRT) and brachytherapy. For EBRT, 1.8–2 Gy fractions were used. If lymph nodes were treated, doses of 45–50 Gy were used. Doses to the prostate were 78–79 Gy. For brachytherapy delivered as a boost, doses were 90 Gy using Pd103 or 100–110 Gy using I125 given after EBRT (45–46 Gy). For brachytherapy used alone, doses were 124 Gy using Pd103 and 145 Gy using I125.
Lovastatin was the HMG-CoA-reductase inhibitor used in this study. If not on a statin, the dose was 20 mg/day PO. Patients already on lovastatin continued the drug at their dose established at the time of enrollment. Patients on a different statin were switched to lovastatin (20–80 mg/day). Lovastatin was started on the first day of radiation and continued for 12 months. If necessary for the management of hyperlipidemia, a statin was continued beyond that point at the discretion of the primary care physician, otherwise it was discontinued.
Patients receiving EBRT were seen at least weekly during treatment and assessed for toxicity. After completing external beam or brachytherapy, all patients were evaluated at Weeks 4 and 8, then Months 4, 6, 9, 12, 15, 18, 21 and 24. At each visit, patients were assessed for toxicity using both patient- and physician-reported instruments.
The primary endpoint of the study was the proportion of physician-reported rectal toxicity ≥ Grade 2 during the first two years after treatment. Patients who did not complete at least six months of lovastatin treatment were deemed ineligible for the purpose of assessing late rectal toxicity and additional patients were enrolled in order to achieve the accrual goal of 53 evaluable patients. The secondary endpoints included erectile function, were also assessed using the validated and reliable EPIC and IIEF instruments as described above with scores compared to baseline at each time point such that each patient would serve as his own control.
Results
Seventy-three consecutive patients enrolled in the study in order to achieve the goal of 53 eligible patients, as 20 patients did not receive at least six months of lovastatin treatment and were ineligible for toxicity assessment.
The median age was 63 years. Thirty-one (58%) were Caucasian and 22 (42%) were African American. The median follow-up was 26 months. Forty-five (85%) were treated with external beam radiation without brachytherapy, two (4%) were treated with external beam radiation plus brachytherapy and the remaining six (11%) were treated with brachytherapy alone. Seventeen patients (32%) received androgen deprivation therapy in conjunction with radiation.
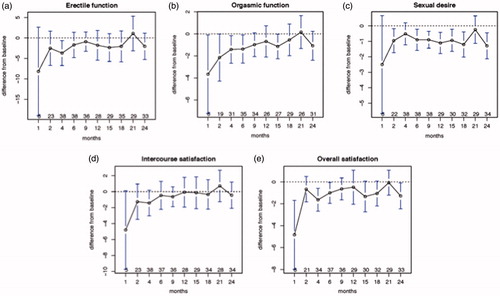
The changes in sexual function over time, as measured by the IIEF, are displayed in . By the first month after treatment, there was a decline across the board in all domains, although the changes were significant only for orgasmic function and overall satisfaction. Improvements were noted in all domains by Month 2. Significant declines from baseline occurred in erectile function at Month 4; in orgasmic function at Months 2, 4 and 6; in sexual desire at Months 2, 6, 9, 12, 15, 18 and 24; and in overall satisfaction at Months 4 and 24. For intercourse satisfaction, although there was a decline compared to baseline at Month 1, at no time point did the change reach significance.
Figure 1. (a) Change in erectile function from baseline with time following the completion of radiation therapy for prostate cancer. (b) change in orgasmic function from baseline with time following the completion of radiation therapy for prostate cancer. (c) change in sexual desire from baseline with time following the completion of radiation therapy for prostate cancer. (d) change in intercourse satisfaction from baseline with time following the completion of radiation therapy for prostate cancer. (e) change in overall satisfaction from baseline with time following the completion of radiation therapy for prostate cancer. The dotted line indicates the baseline. Error bars represent the standard error of the mean ×2.96. Numbers at each time point indicate the number of patients completing this element of the IIEF.
Discussion
To our knowledge, this is the first report of the potential impact of statins on preservation of erectile function following radiation therapy for prostate cancer. The efficacy of lovastatin in this regard varied across the various domains. Lovastatin appeared most successful in preserving intercourse satisfaction and least successful in maintaining sexual desire. Erectile function and orgasmic function seemed to decline early, but was preserved at later time points.
A recent meta-analysis of randomized trials studying the effect of statins on established ED was reported [Citation6]. Eleven studies met the inclusion criteria for the analysis, totaling 713 patients. The most commonly utilized drug was atorvastatin. The median duration of follow-up was only three months. This analysis revealed a statistically significant and clinically relevant improvement in IIEF score by 3.4 points. This magnitude of improvement is about one third to one half of that seen with PDE5 inhibitors [Citation6].
The present study should be considered hypothesis generating only, as the study was not designed nor powered to determine the efficacy of lovastatin in preventing the development of ED after radiation therapy. Also, the study included a heterogeneous group of patients utilizing a range of radiation doses and techniques. In addition, patients were included who had received androgen deprivation, which would be expected to negatively impact on erectile function. Furthermore, the study did not control for possible confounding factors such as diabetes, hypertension and smoking. Also, the drug utilized in the present study, lovastatin, is not very lipophilic, and other statins such as atorvastatin might be more suitable. Nevertheless, the results are intriguing, given that about one third of men report poor sexual functioning at 24 months after radiation [Citation1]. The results of the present study suggest that statins should be studied further as a potential means to prevent radiation-induced ED.
Disclosure statement
The authors declare no conflicts of interest.
References
- Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med 2008;358:1250–61.
- Alicikus ZA, Yamada Y, Zhang Z, et al. Ten-year outcomes of high-dose, intensity-modulated radiotherapy for localized prostate cancer. Cancer 2011;117:1429–37.
- Olsson CE, Alsadius D, Pettersson N, et al. Patient-reported sexual toxicity after radiation therapy in long-term prostate cancer survivors. Br J Cancer 2015;113:802–8.
- Litwin MS, Gore JL, Kwan L, et al. Quality of life after surgery, external beam irradiation, or brachytherapy for early-stage prostate cancer. Cancer 2007;109:2239–47.
- Incrocci L. Radiotherapy for prostate cancer and sexual health. Transl Androl Urol 2015;4:124–30.
- Kostis JB, Dobrzynski JM. The effect of statins on erectile dysfunction: a meta-analysis of randomized trials. J Sex Med 2014;11:1626–35.
- Anscher MS, Chang MG, Moghanaki D, et al. A phase II study to prevent radiation-induced rectal injury with lovastatin. Am J Clin Oncol 2016.
