Abstract
Background: The prognostic value of supraclavicular lymph node (SCN) metastases in esophageal cancer is not well established. We analyzed the prognostic value of SCN disease in patients after definitive chemoradiation (dCRT) for esophageal cancer.
Methods: We retrospectively analyzed 207 patients treated between 2003 and 2013 to identify the prognostic value of metastasis in the SCN on treatment failure and survival. All patients were treated with external beam radiotherapy (50.4 Gy in 28 fractions) combined with weekly concurrent paclitaxel 50 mg/m2 and carboplatin AUC2.
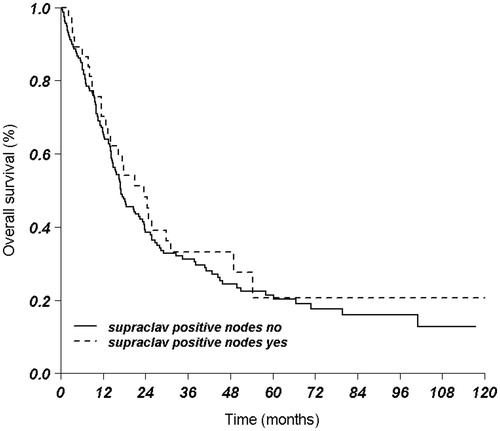
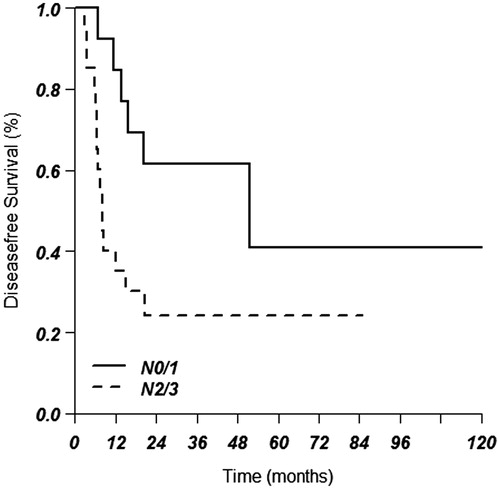
Results: Median follow-up for patients alive was 43.3 months. The median overall survival (OS) for all patients was 17.5 months. OS at one, three and five years was 67%, 36% and 21%, respectively. For patients with metastasis in a SCN, OS was 23.6 months compared to 17.1 months for patients without metastasis in the SCN (p = .51). In multivariate analyses, higher cT status, cN status and adenocarcinoma were found to be prognostically unfavorable, but a positive SCN was not (p = .67). Median OS and median disease-free survival for tumors with SCN involvement and N0/1 disease was 49.0 months and 51.6 months, respectively, compared to 14.2 months and 8.2 months, respectively, in patients with N2/3 disease.
Conclusion: In esophageal cancer treated with dCRT, the number of affected lymph nodes is an important independent prognostic factor, whereas involvement of a SCN is not. Supraclavicular lymph nodes should be considered as regional lymph nodes and treated with curative intent if the total number of involved lymph nodes is limited.
Introduction
Patients with esophageal cancer have a poor prognosis with a five-year survival rate of about 15% [Citation1]. Patients with a resectable stage of disease and treated with preoperative chemoradiation (CRT) and surgery have a better prognosis, i.e. a five-year survival of up to 48% [Citation2]. Inoperable or irresectable non-metastasized patients referred for definitive chemoradiation (dCRT) have a five-year survival rate of only 20–25% [Citation3,Citation4]. Thus, adequate treatment selection seems crucial.
In the TNM 7 classification, metastases in the supraclavicular lymph nodes (SCN) are considered as distant metastasis (M) and thus prognostically unfavorable [Citation5]. Metastatic disease is generally considered incurable and is, therefore, not generally accepted for radical surgical treatment. In various surgical series, tumor-related prognostic factors for worse outcome include higher T stage, higher N stage and higher M stage, including SCN [Citation6–9]. Most of these series are in patients without any (neo)adjuvant therapy and only in squamous cell carcinoma. However, the independent prognostic value of a SCN metastasis has recently been questioned [Citation10].
In patients treated with dCRT, N stage disease is considered a prognostic factor. However, data on the independent prognostic value of SCN metastasis (considered as M1) is both limited and conflicting [Citation3,Citation11].
The present study retrospectively analyzed patients in a single tertiary referral center for esophageal cancer, treated with a standardized protocol of dCRT, to identify the prognostic value of metastasis in the SCN on treatment failure and survival.
Methods
Patients
A total of 207 patients were identified who were treated with dCRT for esophageal cancer at the Academic Medical Center Amsterdam from January 2003 to December 2013. Data was retrieved in March 2015, ensuring a minimum potential follow-up of 15 months. All patients had a histologically confirmed esophageal carcinoma and no distant metastases on computed tomography (CT) scan and positron emission tomography (PET) scan. Indication for dCRT was supraclavicular disease, T4b stage or otherwise irresectable, medical inoperability and patients refusing surgery. Data on treatment and outcome were retrieved from the clinical chart and retrospectively recorded in an electronic database.
T1 and T2 were analyzed as one group because of the small numbers in T1. Similarly, because of the relatively small numbers in the N0 and N3 subgroups, the N stage was analyzed both separately and divided into two groups, N0/1 and N2/3. Toxicity was scored using the Common Terminology Criteria for Adverse Events (CTCAE version 4.0). In case of multiple toxicities, the highest score is reported.
Histology of adenocarcinoma, squamous cell carcinoma, undifferentiated carcinoma and large cell carcinoma were included. Patients treated for recurrent disease were excluded from the analyses. Treatment consisted of a standardized protocol in all patients: a radiation dose of 50.4 Gy in 28 fractions of 1.8 Gy, 5 fractions per week, combined with weekly chemotherapy starting on the first or second day of radiotherapy. External beam radiation was applied using a three-dimensional (3D) conformal radiation technique or intensity modulated radiation therapy (IMRT). Chemotherapy consisted of weekly concurrent paclitaxel 50 mg/m2 and carboplatin targeted at an area under the curve of two, six courses in total. Patients were included in this analysis if dCRT was started, irrespective of completion of therapy. Patients were excluded if a radiation boost dose was given.
Identification of supraclavicular lymph nodes
SCN metastasis was defined as follows: pathologically confirmed and situated between the inferior belly of the omohyoid muscle posteriorally, the clavicle/upper border of the manubrium anteriorly, and inferiorly and cranially inferior to the lower margin of the cricoid.
In four patients, no cytology of the suspicious SCN was obtained. In one patient a biopsy was impossible due to the proximity of critical vessels, in one patient the SCN was very close to the primary tumor and would therefore be included in the target volume, in one patient the biopsy was inconclusive, and in one patient no biopsy was performed because imaging was deemed sufficient. In all four patients, imaging was very suspicious for metastatic disease.
Radiation fields
For treatment simulation and planning purposes, all patients had 3D CT or PET/CT scanning with oral contrast. Treatment position was supine, with the arms raised above the head. For patients with a proximal tumor, a fixation mask was used and arms were placed next to the body. The gross tumor volume (GTV) was contoured on the planning CT scan by the radiation oncologist, using data from PET/CT fusion scans when available, endoscopic ultrasound reports and diagnostic CT images. The clinical target volume (CTV) consisted of GTV plus the peri-esophageal lymph node area extended in cranio-caudal direction by 3.5 cm margin. CTV was extended in case of pathological nodes outside the 3.5 cm region. The cranial border of the CTV was restricted to the SCN area if the tumor was limited to the upper mediastinum. If a SCN was involved, the CTV was expanded to include the contralateral supraclavicular region. The CTV-planning target volume (PTV) margin was 1.0 cm in all directions for mid and distal tumors and 8 mm for proximal tumors combined with the use of a fixation mask. Radiation was delivered as a four-field conformal beam arrangement, or IMRT with the following normal tissue constraints: <30% volume of the lungs receiving 20 Gy; <50% of the lungs receiving 10 Gy, <50% volume of the heart receiving 45 Gy; <10% volume of the spinal cord receiving 50 Gy (Dmax <50 Gy); <25% volume of one kidney receiving 20 Gy; and <30% volume of the liver receiving 40 Gy.
Follow-up
A CT scan was carried out eight weeks after completion of dCRT to assess response, which also served as the baseline for follow-up. All patients were reviewed clinically every three months for one year, every six months in the second and third year, and thereafter once yearly. Follow-up consisted of clinical evaluation and physical examination. CT scan, PET scan or endoscopic examination were performed on indication.
Locoregional recurrences after dCRT were defined by clinical signs (e.g. progressive dysphagia, and/or retrosternal pain) combined with progression on CT scan or PET/CT scan, or suspicious endoscopic findings and/or histological proof of recurrence. Histological confirmation of a recurrence after dCRT was present in most cases, but omitted if a local recurrence was obvious based on clinical symptoms and PET/CT or endoscopy. The sites of locoregional recurrence were reconstructed to the radiation fields and scored as in-field or out-field (related to the 95% isodose line/PTV). Distant metastases were scored separately. The date of recurrence was defined as the date of proven histology (if present) or the date of imaging of recurrent disease.
Statistical analysis
Continuous and categorical variables were summarized by descriptive statistics. Overall survival (OS) and local recurrence rate were estimated from the start of dCRT until death and local recurrence or the last follow-up using the Kaplan-Meier methodology. Univariate and multivariate Cox proportional hazards models were fit to evaluate the impact of factors predictive of OS, and locoregional recurrence-free survival and disease-free survival. Analyses were performed with SPSS version 20.0 (IBM Corporation, Armonk, NY).
Results
Patient characteristics
A total of 207 patients met the inclusion criteria. Of these, eight were excluded from the analyses because information on the N status was unavailable and two patients were excluded because the T stage was not available. Thus, a total of 197 patients were analyzed; the characteristics of these patients are presented in . For the entire cohort, median follow-up was 16.3 (range 0.1–120) months. Median follow-up for patients alive was 43.3 (4.4–120) months. Only four patients were lost to follow-up after 4, 33, 36 and 64 months, respectively. All other patients were followed until death or the end of the study analyses (December 2013).
Table 1. Characteristics of the study population (n = 197).
Survival
The median OS for all patients was 17.5 months. The one, three and five-year OS was 67%, 36% and 21%, respectively. The median OS was 22.0 months for the squamous cell carcinoma group and 14.9 months for the adenocarcinoma group (p = .02).
Of the 197 patients, during follow-up 83 (42%) developed a locoregional recurrence. Median locoregional recurrence-free survival was 27.3 months, and the one, three and five-year locoregional recurrence-free survival was 69%, 48% and 44%, respectively. Median disease-free survival was 16.8 months, and the one, three and five-year disease-free survival was 60%, 39% and 31%, respectively.
Risk factors for overall survival
In univariate analyses only histology of adenocarcinoma (p = .01) and higher N stage (N0/1 vs. N2/3, p = .018) were significantly associated with worse outcome. The influence of a higher T stage (p = .23), the absence of a positive SCN (p = .58), female sex (p = .75), and tumor location in distal esophagus with cardia involvement (p = .07) were not statistically significant.
Multivariate analyses on survival
Multivariate analyses were performed including the following factors: age, sex, tumor location, adenocarcinoma histology, T stage, N stage, SCN involvement, and tumor length (). Age and tumor length were analyzed as a continuous variable. T4 was used as reference category in the analyses, therefore a hazard ratio <1 for T stage indicates a reduced risk for death compared to T4 disease. Because of the few patients with either N0 or N3 disease, N1 stage was used as reference.
Table 2. Multivariate analyses of risk factors for worse overall survival.
Subgroup supraclavicular lymph node involvement
For patients with a metastasis in the SCN, median OS was 23.6 months compared to 17.1 months for patients without a metastasis in the SCN (p = .51) ().
The relationship between SCN involvement and N stage (N0/1 vs. N2/3) was analyzed separately.
Median OS for tumors with SCN involvement and N0/1 disease was 49.0 (CI 15.4–82.7) months compared to 14.2 months in the N2/3 group (CI 8.5–20, p = .08). Median disease-free survival for tumors with SCN involvement and N0/1 disease was 51.6 (95% CI 0–108.5) months compared to 8.2 months in the N2/3 group (95% CI 6.2–10.1, p = .015) ().
Conversely, in N0/1 patients, median OS was 22 (95% CI 15.7–28.4) months in the SCN negative group and 49 (95% CI 15.4–82.7) months in the SCN positive group. In the N2/3 group, median OS was 14.6 (95% CI 12.5–16.6) months in the SCN negative group and 14.2 (95% CI 8.5–20.0) months in the SCN positive group (p = .81).
Toxicity
Nearly all patients (92%) completed radiotherapy and the majority (75%) completed all cycles of chemotherapy.
Acute toxicity is reported in . Grade 1–3 toxicity consisted mainly of dysphagia and swallowing pain. Seven patients (3%) had grade 5 toxicity: two patients died of cardiac causes and had a history of prior cardiac disease, one patient died of an ileus based on (missed) abdominal metastasis, one after esophageal bleeding, one after neutropenic sepsis, one after aspiration due to perforation, and one patient died of unknown cause one month after treatment. Of these latter patients, only one had a positive SCN, all but one died before the end of radiotherapy, and five of these patients were aged ≥75 years. There was no significant difference in toxicity between patients with an involved SCN and those without. Also, in patients with an involved SCN, in those with and in those without the tumor located distally in the esophagus, no significant differences were observed.
Table 3. Data on acute toxicity.
Discussion
A positive SCN was not of independent prognostic value for survival in patients referred for dCRT in either the univariate or multivariate analysis. This is in contrast with the most recent TNM (7th edition) staging, in which supraclavicular localization is defined as M1 disease and is not part of the N stage, thereby suggesting to be of extra negative prognostic value [Citation12]. In agreement with the literature, histology, the cT stage and the cN stage were found to be important prognostic factors after dCRT for esophageal cancer. The N stage remains an important prognostic factor in the group of patients with an involved SCN; in this subgroup, although not yet significant for OS (p = .097), the N stage is significant for disease-free survival (p = .028). Furthermore, the presence or absence of a SCN metastasis does not affect the lower OS in patients with an N2/3 disease.
Therefore, in patients referred for dCRT, we suggest that a positive SCN should be regarded as just another regional node and as part of the N stage, and not as an independent prognostic factor.
In our patients with involvement of the SCN, treatment did not result in any increase of acute toxicity. Even in patients with a distally located tumor and SCN involvement (indicating large radiation fields), acute toxicity remained similar to that in patients without SCN involvement. An explanation for this may be that radiation fields in dCRT are always relatively large due to the large GTV or high N stage, including the GTV to CTV margin of 3.5 cm. The generally large PTV in dCRT leads to substantial acute toxicity, without much extra toxicity due to inclusion of the SCN area. Treatment in the present study was generally well tolerated, with dysphagia and swallowing pain as the most common toxicities. Nearly all patients (92%) completed radiotherapy and the majority (75%) completed all cycles of chemotherapy. Grade 5 toxicity was rare (3%) and mainly occurred in elderly patients.
TNM 6 to TNM 7
In the seventh edition of the TNM (AJCC), the staging of esophageal cancer has undergone major changes. All paraesophageal lymph nodes are considered regional, thus a celiac lymph node is no longer scored as a metastasis. A further differentiation is made in the number of lymph nodes. These have been designated N0 (none), N1 (1–2 nodes), N2 (3–6 nodes) and N3 (≥7 nodes), and are identical to the gastric N classifications. Furthermore, a SCN metastasis is scored as M1 [Citation12]. This new edition of the TNM was based on 4627 operated patients who received no (neo) adjuvant therapy [Citation5,Citation12]. In this latter group, mean tumor length was small (3.3 ± 2.5 cm) and only 23% of patients had more than two positive lymph nodes, the current N2/N3 disease. As this was a surgical series, M1 patients due to SCN involvement were scarce (7.8%); the authors stated that their data are not representative for the M1 patient group. Their difference in survival between M1 and M0 showed a remarkable resemblance to the difference they report between node positive and node negative patients, which is in agreement with our current data. Unfortunately, they did not report any multivariate analyses, which may have shown that a positive SCN is simply an indicator for N + disease. Overall, their conclusion that a positive SCN is an isolated risk factor for disease does not seem to be well founded. In the present series, the OS of the SCN positive patients was 23.6 months with a five-year survival of 20%, which is considerably higher than the 8–13 months reported in M + disease based on distant metastasis [Citation1,Citation13]. This further suggests that SCN positivity does not reflect the prognostic impact of M + disease.
Peyre et al. investigated the relationship between the number of involved lymph nodes and the likelihood of distant metastases. They found that the risk for development of systemic disease at five years was related to the number of involved nodes: being 16% in patients without nodal involvement, progressing with each extra node involved to 93% when eight or more nodes were involved [Citation7]. The relationship was non-linear, with the largest increase of risk occurring with the first number of involved lymph nodes. In accordance with Peyre et al., Chen et al. also found that the number of lymph nodes (0, 1, 2–3, and ≥4 positive lymph nodes) better represented survival outcome than the TNM 7 node grouping [Citation9].
In Japan, it has been policy to perform a three-field esophagectomy in selected patients, which includes the bilateral SCN. Tachimori et al. retrospectively analyzed 1309 patients who underwent a three-field esophagectomy and had an R0 resection in mainly squamous cell carcinoma tumors [Citation10]. In univariate analysis they indeed found metastasis in a SCN to be prognostically unfavorable (p < .001) which was almost significant in multivariate analysis (p = .062). However, when they considered a SCN as an extra regional lymph node, thereby changing the M status to M0 and affecting the N status, there was no longer any difference. Another Japanese series showed similar findings in a cohort of 665 patients [Citation14].
In summary: in esophageal cancer, the SCN seems to be just another regional lymph node level and should be staged and treated as such. However, the number of involved lymph nodes remains of prognostic value. On the other hand, it is debatable whether patients with N3 disease and M0, bearing a very poor prognosis, should be treated with curative intent, irrespective of the involvement of the supraclavicular node.
Limitations
This study has some limitations. It is retrospective and data are from a single institution. Furthermore, lymph nodes were only scored per stage (e.g. N0, N1) and not counted separately. Also, because we did not analyze the size of the radiation fields, correlations between tumor location and toxicity are approximations only.
Strengths include the large number of patients from a large referral center for esophageal cancer, a uniform treatment regime for all patients, and the availability of long-term follow-up data recorded with a standard protocol. The presented data are from one of the few series on the prognostic value of lymph node extent and localization after treatment with dCRT.
Conclusion
In esophageal cancer treated with dCRT, involvement of a SCN is not an independent prognostic factor. Classification of supraclavicular lymph node metastasis as M + in the current TNM classification does not reflect the relatively good prognosis compared to M + disease based on distant metastasis. However, the number of affected lymph nodes is an important factor. The supraclavicular lymph node should be considered as a regional lymph node and treated with curative intent if the total number of involved lymph nodes is limited, irrespective of the site of the primary tumor.
Disclosure statement
The authors report no conflicts of interest.
References
- Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet 2013;381:400–12.
- van Hagen P, Hulshof M, van Lanschot J, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer for the CROSS Group. N Engl J Med 2012;366:2074–84.
- Versteijne E, van Laarhoven HWM, van Hooft JE, et al. Definitive chemoradiation for patients with inoperable and/or unresectable esophageal cancer: locoregional recurrence pattern. Dis Esophagus 2015;28:453–9.
- Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA 1999;281:1623–7.
- Rice TW, Rusch VW, Apperson-Hansen C, et al. Worldwide esophageal cancer collaboration. Dis Esophagus 2009;22:1–8.
- Bus P, Lemmens VE, van Oijen MG, et al. Prognostic factors for medium- and long-term survival of esophageal cancer patients in the Netherlands. J Surg Oncol 2014;109:465–71.
- Peyre CG, Hagen JA, DeMeester SR, et al. Predicting systemic disease in patients with esophageal cancer after esophagectomy: a multinational study on the significance of the number of involved lymph nodes. Ann Surg 2008;248:979–85.
- Talsma K, van Hagen P, Grotenhuis BA, et al. Comparison of the 6th and 7th Editions of the UICC-AJCC TNM classification for esophageal cancer. Ann Surg Oncol 2012;19:2142–8.
- Chen S-B, Weng H-R, Wang G, et al. Prognostic factors and outcome for patients with esophageal squamous cell carcinoma underwent surgical resection alone: evaluation of the seventh edition of the American Joint Committee on Cancer staging system for esophageal squamous cell carcinoma. J Thorac Oncol 2013;8:495–501.
- Tachimori Y, Ozawa S, Numasaki H, et al. Supraclavicular node metastasis from thoracic esophageal carcinoma: a surgical series from a Japanese multi-institutional nationwide registry of esophageal cancer. J Thorac Cardiovasc Surg 2014;148:1224–9.
- Haefner MF, Lang K, Krug D, et al. Prognostic factors, patterns of recurrence and toxicity for patients with esophageal cancer undergoing definitive radiotherapy or chemo-radiotherapy. J Radiat Res 2015;56:742–9.
- Sobin LH, Compton CC. TNM seventh edition: what's new, what's changed: communication from the International Union Against Cancer and the American Joint Committee on Cancer. Cancer 2010;116:5336–9.
- Cunningham D, Okines AFC, Ashley S. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2010;362:858–9.
- Yamasaki M, Miyata H, Miyazaki Y, et al. Evaluation of the nodal status in the 7th edition of the UICC-TNM classification for esophageal squamous cell carcinoma: proposed modifications for improved survival stratification: impact of lymph node metastases on overall survival after esophagectomy. Ann Surg Oncol 2014;21:2850–6.