Abstract
Background: Previous studies have shown an increase in the incidence of cutaneous melanoma (CM) in Estonia, but also poor survival in international comparisons, with a significant survival gap between the sexes. The aim of this study was to analyze the time trends in CM incidence and relative survival by age, TNM stage and anatomical subsite among men and women in Estonia.
Materials and methods: Data from the Estonian Cancer Registry were used to calculate age-standardized (World) and age-specific incidence of CM in 1995–2013, and five-year relative survival ratios (RSR) for cases diagnosed in 1995–2012 and followed through in 2014. Period hybrid analysis was used to calculate the most recent survival estimates for 2010–2014.
Results: Between 1995 and 2013, the age-standardized incidence of CM increased significantly in Estonia among both sexes, at a rate of around 4% per year. Among women, the proportion of trunk melanomas increased from 26% in 1995–1999 to 39% in 2010–2012 and became the most common site. The proportion of stage I cases and T1 tumors increased considerably. Women had more favorable stage distribution and thinner tumors than men. The age-adjusted five-year RSR increased significantly, from 64% in 1995–1999 to 81% in 2010–2014. The latest age-adjusted RSRs were 76% among men and 84% among women. Survival gains were the largest in patients below 50 years, those with head and neck or trunk melanomas, and stage III cancer.
Conclusions: The proportion of stage I and T1 cases is lower in Estonia compared with the Scandinavian data and is likely a major contributor to the persisting overall survival deficit in Estonia. The apparent deficit in stage II survival also warrants further investigation. A public health program is necessary in Estonia to raise awareness of CM and to significantly increase early stage diagnosis.
The incidence of cutaneous melanoma (CM) is rising in Caucasian populations around the world [Citation1]. The same applies to Estonia, as cancer incidence and survival analyses have shown increased incidence of CM, but also poor survival in international comparisons [Citation2,Citation3]. According to the EUROCARE-5 study, the European mean age-standardized five-year relative survival (RS) of patients diagnosed with CM during 2000–2007 was 83%, but only 72% for Estonia [Citation4]. The European mean RS was significantly higher in women than men; similar results were evident for patients in all age groups and in each European region. In Estonia, the gap between the sexes has been remarkably wide. An analysis of CM cases in Estonia diagnosed in 1995–2006 showed that the absolute difference between the five-year RS of male and female melanoma patients increased from 14% units to 19% units from 1995–2000 to 2001–2006 as survival did not improve in men [Citation5]. The five-year RS for women in Estonia in 2001–2006 was 12% units lower than the European mean for 2000–2007 reported in EUROCARE-5 (75% vs. 87%), but the respective difference among men was as much as 23% units (56% vs. 79%) [Citation5].
There are many prognostic factors which affect the treatment outcomes and survival of patients with malignant melanoma. CM survival is strongly related to stage at diagnosis as thinner CM have a better prognosis than thicker ones [Citation6]. Other prognostic factors include age, skin type, lifestyle, pathomorphological characteristics of the tumor, genetic mutations and gender-related physiological variations [Citation7]. Also, access to health services, quality of medical care and time from diagnosis to treatment play an important role in survival [Citation4].
The aim of this study was to analyze time trends in CM incidence, changes in age, stage and subsite distribution, and RS by age, stage and subsite among men and women with CM in Estonia during 1995–2014. To our knowledge, this is the first study using TNM stage for CM in Estonia.
Materials and methods
The study used data collected by the Estonian Cancer Registry (ECR), a population-based registry that covers the whole country (population 1.34 million in 2011) and has had complete nationwide coverage since 1968. Reporting to the registry is mandatory by law for all physicians and pathologists in Estonia who diagnose or treat reportable tumors. Multiple sources are used for case ascertainment, including trace-back of cases first identified via death certificates as well as linkages with the databases of cancer centers. The percentage of death certificate only (DCO) cases has been stable and around 2% for all sites and 0.6% for CM [Citation2]. The data quality indicators have been similar for male and female cases [Citation5]. The registry uses ICD-O-3 for coding and follows international definitions and rules, including those for multiple primaries, issued by the European Network of Cancer Registries and the International Association of Cancer Registries.
For survival analysis, the ECR provided data on all cases of primary invasive CM (ICD-10 code C43) diagnosed in adults (age 15 years and over) in Estonia during 1995–2012 (n = 2645), regardless of cancer sequence. After the exclusion of DCO and autopsy cases (n = 25), 2620 cases were included. The cancer notification forms used for data collection were uniform during the whole study period. The patients were followed up until 31 December 2014 using linkage to the Estonian Population Registry. This routine linkage is based on unique personal identification numbers. The Population Registry provided information on the vital status of the patients as well as the date of death/emigration. Based on ICD-O-3 topography codes, tumor locations were grouped into head and neck (C44.0–44.4), trunk (C44.5), upper limb and shoulder (C44.6), lower limb and hip (C44.7), and other (overlapping and unspecified, C44.8–44.9). Pathological or clinical TNM stage is reported to the ECR by clinicians according to current TNM classification. For this study, all cases were reviewed and summary stage was compiled based on the Union for International Cancer Control version 7 of the TNM classification [Citation8]. Category unknown includes cases with no or incomplete information on stage. Age at diagnosis was categorized into five groups: 15–49, 50–59, 60–69, 70–79, and ≥80 years. Mortality data were obtained from the Estonian Causes of Death Registry and population denominator data from Statistics Estonia.
The significance of the difference between proportions was tested using chi-square test. Relative survival ratios (RSR) were calculated as the ratio of the observed survival of the CM patients and the expected survival of the underlying general population. The latter estimate was calculated according to the Ederer II method [Citation9] using national life tables stratified by sex, single year of age and calendar year. We used cohort analysis for patients diagnosed in 1995–1999, 2000–2004 and 2005–2009, and period estimation for 2010–2014 in order to detect the most recent survival trends [Citation10]. As follow-up was more up-to-date than the registration of incident cases, a modification of period analysis, called hybrid analysis was used [Citation10]. The International Cancer Survival Standard (ICSS) population was used for age-standardizing RS estimates [Citation11]. All calculations were conducted with Stata 14.1 (StataCorp LP, College Station, TX); survival analysis was performed using the strs module [Citation12].
Time trends in age-specific and -standardized (World standard population) CM incidence (1995–2013) and mortality (1995–2014) were analyzed and the estimated annual percentage change (APC) with 95% confidence intervals calculated with Joinpoint Regression Program (version 4.1.1.1) from the Surveillance Research Program of the US National Cancer Institute (http://surveillance.cancer.gov/joinpoint/).
The study protocol was approved by the Research Ethics Committee of the University of Tartu.
Results
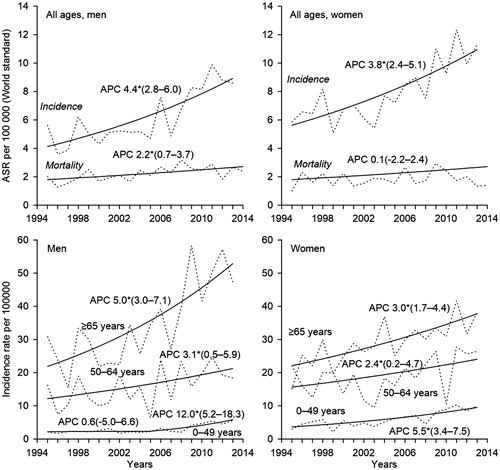
Since 1995, the age-standardized incidence of CM has increased significantly in Estonia among both sexes, at a rate of around 4% per year (). The increase has been slightly steeper among men. In 2013, the age-standardized incidence of CM in Estonia was 8.6 and 11.3 per 100 000 person-years among men and women, respectively. The age-standardized mortality rate increased only among men (APC 2%); in 2014, the respective rates were 2.4 and 1.4 per 100 000 among men and women, respectively. Time trends of CM incidence varied by age group. A statistically significant increase of 12% per year appeared among men younger than 50 years after 2005, as the incidence rate increased from 2.2 per 100 000 in 2005 to 5.5 per 100 000 in 2013. A steady and also statistically significant increase of 3% and 5% per year was observed over the whole study period among men aged 50–64 years and ≥65 years, respectively. As a result, the difference between the youngest and oldest age group increased considerably. The differences between age groups were smaller among women. The largest increase over the study period was seen among women below 50 years (APC 6%), although a smaller, yet significant increase was seen among older age groups.
Figure 1. Observed (dashed line) and modeled (solid line) age-standardized (World standard population) rates, age-specific incidence rates and annual percent change (APC) with 95% confidence intervals for trends in cutaneous melanoma incidence (1995–2013) and mortality (1995–2014) in Estonia. *The APC is significantly different from zero at alpha = 0.05.
Among the 2645 cases of primary CM diagnosed among adults in Estonia in 1995–2012, 17 (0.6%) were DCO cases and eight (0.3%) diagnosed at autopsy. Among the 2620 cases included in survival analyses, 966 (37%) were men and 654 (63%) women. Median age at diagnosis was 63 years for both sexes.
The age distribution changed slightly over the study period for both sexes (). A large change occurred in the subsite distribution among women as the proportion of trunk CM increased from 26% in 1995–1999 to 39% in 2010–2012 (p = .0001). There was a significant shift in the stage distribution as the proportion of stage I cases increased considerably and the proportion of stage II disease decreased accordingly. No particular changes appeared in the proportion of other stages. Similarly, there was a significant shift towards thinner tumors. The comparison of stage distribution between the sexes demonstrated a significantly lower proportion of stage I cases among men during all periods; similarly, the proportion of T1 cases was consistently lower among men.
Table 1. Distribution of cutaneous melanoma cases by age, subsite and stage at diagnosis, Estonia 1995–2012.
The age-adjusted five-year RSR increased significantly over the study period, from 64% in 1995–1999 to 81 in 2010–2014 (). A significant change of 21% units was seen among men (from 55% to 76%) and of 15% units among women (from 69% to 84%). Survival increase over the study period was the largest in patients below 50 years at diagnosis (21% units) and those with head and neck or trunk melanomas (25% units and 22% units, respectively). The largest change by stage occurred among stage III patients (21% units).
Table 2. Five-year relative survival ratio for cutaneous melanoma by sex, age, subsite and stage, Estonia 1995–2014.
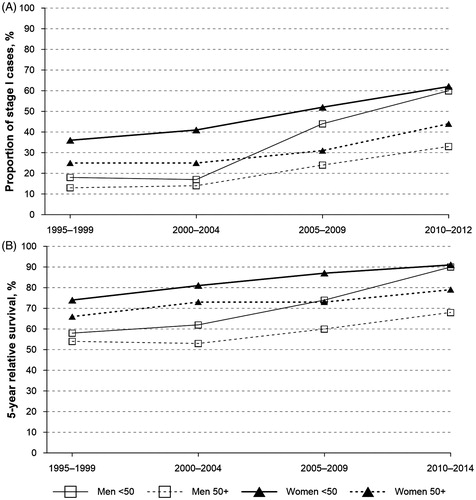
shows how the proportion of stage I tumors has altered among men and women in different age groups (<50 years and ≥50 years) and the corresponding increase in the five-year RSRs. The largest change both in the proportion of stage I cases as well as the five-year RSR was apparent among men younger than 50 years at diagnosis.
Figure 2. Proportion of stage I cases (A) and 5-year relative survival (B) by sex and age in Estonia, 1995–2014.
displays the five-year RSRs by sex and stage during different periods. The five-year RSR for stage I was around 100% for both sexes during the latest period. Survival for stage II disease increased only among women, whereas the largest increase appeared for male stage III patients.
Table 3. Five-year relative survival ratio for cutaneous melanoma by stage, men and women, Estonia 1995–2014.
Discussion
In this population-based study based on cancer registry data, we found an increase in the incidence and survival of CM in Estonia during 1995–2014. Mortality increased only among men. We also observed significant changes in the distribution of primary CM by anatomical subsite, TNM stage and tumor thickness. The survival gap between men and women decreased over the study period, but remained wide among patients aged 50 years and older. At the same time, the age gradient increased, as younger patients experienced a larger survival gain than older patients.
The major strength of this study was the use of high quality data from the ECR – for CM, 99% of cases were microscopically verified and below 1% were DCO cases [Citation2]. Another strength of the study was the availability of data on TNM stage, which is more accurate than the commonly used classification of tumors into localized and advanced stage. Our study was limited by the possible misclassification of stage due to some missing data on T subcategories as well as the lack of data on histopathological characteristics and treatment.
The incidence of CM has risen fast in European countries since the middle of 1950s [Citation13]. Although it has been questioned whether this could be due to overdiagnosis or earlier and more accurate detection of early stage CM − partly as a result of prevention campaigns carried out in many countries with the aim of raising population awareness − the trends observed in several studies suggest true increase in incidence [Citation14]. It is known that the main risk factor for CM is the history of intermittent exposure of untanned skin to high intensity UV radiation. Changes in risk behavior, including a combination of increased sun exposure, exposure to artificial UV light, as well as changes in clothing style, are the likely explanations for rising incidence trends. Intensive UV exposure in childhood and adolescence also increases the risk of developing CM during lifetime [Citation15]. Risk rises with more than 100 atypical nevi and a family history of melanoma. It has been found that Caucasian populations have a higher prevalence of atypical nevi [Citation16]. The number of newly diagnosed cases in men in Estonia increased from 41 cases per year in 1995–1999 to 82 cases per year in 2010–2012; among women, the respective numbers were 75 and 119. Although the incidence of CM has been increasing in Estonia since the 1970s, the rise has been particularly steep since the 1990s, after the country’s transition to open market economy, which was accompanied by changes in risk behavior, including increased accessibility and affordability of spending holidays in sunny locations, and use of tanning beds. A recent health behavior survey revealed that 30% of young women aged 16–24 had used tanning beds in the past 12 months; and close to 30% of all respondents aged 16–64 had experienced sunburn in the past 12 months [Citation17]. The age-standardized incidence of CM in Estonia, as well as the rates in ages 50 years and older, are approximately two times lower compared to the Nordic countries [Citation18]. However, as a result of the recent rapid incidence increase in men and women below 50 years in Estonia, the incidence in this age group has become very close to that seen in the Nordic countries for both sexes [Citation17]. Many studies have reported an association between anatomic location of CM and prognosis, showing that lesions on the extremities have a better prognosis than those on the head, neck or trunk [Citation7]. In our study, we found that among men, the trunk was the most common anatomical site across the entire study period, whereas among women, lower limbs were replaced by trunk as the most common subsite by the latest period. The increasing incidence of trunk melanomas has been observed also in other studies [Citation4,Citation13].
The main contributors to the increasing trend in incidence in many countries have been shown to be thinner, less invasive and localized melanomas [Citation19]. We observed a significant shift towards earlier stage and thinner tumors in both sexes. Overall, the proportion of stage I cases remained higher among women during the whole period. However, among male patients younger than 50 years, the proportion of stage I cases increased rapidly and equaled that among women by the latest period. This change occurred in parallel with a steep increase in incidence and could be related to sudden increase in population awareness as a result of the melanoma death of a young male popular artist, which received a lot of media coverage. Still, the proportion of stage I cases remained well below the proportion observed in Denmark in the same age group; and the discrepancy was even larger when comparing stage distribution in patients 50 years and older [Citation20]. The proportion of T1 tumors increased over time both in male and female patients. However, the comparison of our data with the findings of a Swedish study showed that the proportion of T1 tumors among those with known T-category was higher in Sweden in 2007–2011 than in Estonia in 2010–2012: 51% versus 40% among men and 57% versus 48% among women, respectively, whereas the respective proportions of T4 tumors were 13% versus 27% among men and 10% versus 19% among women (data not shown) [Citation21].
Our study showed a significant improvement in five-year RS from 64% (95% CI 59–69) in 1995–1999 to 81% (95% CI 77–84) in 2010–2014. The latter estimate, however, is still lower than the European mean of 85% for 2005–2007 [Citation22]. Significant increase was seen both among men (from 55% in 1995–1999 to 74% in 2010–2014) and women (from 68% in 1995–1999 to 82% in 2010–2014). Still there is a differentiable gap between Nordic countries. For example, the Nordcan data shows that five-year RS in Sweden during the period 2010–2014 is 89% for men and 94% for women [Citation17]. The difference in sex-specific age-adjusted RSRs decreased from 14% units to 8% units over the study period. Gender disparities in melanoma survival may be associated with different stage and subsite distribution as well as health behavior between the sexes. There is presumably better health care service utilization among women [Citation3]. However, it has also been suggested that biological differences between the sexes may be equally important. For example, it has been shown that women have thinner lesions with less frequent ulceration compared to men [Citation7]. Studies have found a protective effect of the female sex even after adjusting for a number of tumor characteristics [Citation23]. The survival advantage for female patients may be associated with the sex chromosome gene expression, hormonal regulation, oxidative stress response, vitamin D metabolism, immune function differences between the sexes [Citation24]. A previous analysis in Estonia found a significant survival gap between men and women after adjusting for age, extent of disease and subsite [Citation5]. Among patients younger than 50 years in our study, the gender gap had disappeared by the latest period, which is consistent with the trends seen in stage distribution.
Age is an important predictor of survival. In EUROCARE-5, the difference in European mean five-year RS between the youngest (15–44 years) and oldest (≥75 years) patients was 18% units, and fairly similar across the different European regions [Citation22]. In our study, younger patients experienced faster improvement in survival than older patients and thus the age differences increased. Many studies have shown that older patients have poorer prognosis due to thicker and more ulcerated melanomas [Citation7]. In patients younger than 50 years at diagnosis, the proportion of stage I tumors increased to 61% by the latest period (68% among those with known stage), but only to 31% (36%) among those older than 70 years (data not shown). Elderly individuals therefore depart from the younger age group – the latter show a trend towards early detection. Furthermore, blurred vision and awareness, decreased flexibility, or lack of motivation to visit a general practitioner makes it difficult to detect melanoma as early as in stage I [Citation25].
The overall five-year RSR in 2010–2014 remained 6% units lower in Estonia than in Denmark several years earlier among both sexes (), whereas the largest deficit of Estonian patients (12% units) was seen for stage II disease. One possible explanation for this difference is different histopathological characteristics of stage II tumors, which we were not able to study. Breslow thickness, ulceration, mitotic rate and metastasis are described as the major prognostic factors among CM patients [Citation26]. The development of ulceration has been shown to be associated with higher incidence of metastasis and thus, worse prognosis. Studies have shown that the incidence of ulceration rises with increasing tumor thickness, ranging from 6%–12.5% for thin melanomas to 63%–72.5% for thick lesions [Citation7]. Stage migration should be considered as another possible reason behind the stage-specific survival differences. Unfortunately, no data were available to investigate the thoroughness of diagnostic workup and the possibility of undetected lymph node or distant metastasis. Similarly, we were not able to look at treatment practices.
Table 4. Stage distribution and age-adjusted five-year relative survival ratio by stage in Estonia (2010–2014) and Denmark (2004–2008)a.
In conclusion, we observed rapid increase in the incidence of CM in Estonia, which was accompanied by considerable survival improvement. Significant changes were apparent in CM distribution by anatomical site, TNM stage and tumor thickness, which influence the prognosis of melanoma patients and have potentially different impact on the survival of men and women. We observed converging trends for male and female patients younger than 50 years at diagnosis, consistent with changes in stage distribution. However, the survival of CM in Estonia remains lower than in other European countries; this can be explained to a large extent by differences in proportions of early diagnosis. The apparent deficit in stage II survival warrants further investigation and evaluation of the quality of care, including surgical performance and pathology. In order to reduce CM incidence and mortality in Estonia, a vigorous public health program is necessary to achieve favorable changes in risk behavior and reduce UV exposure. Increased public and professional awareness of CM, particularly in the primary care setting, and improved self-examination would help to promote early stage diagnosis in all age groups.
Acknowledgements
The authors thank Dr Margit Mägi and Mrs Pille Härmaorg from the Estonian Cancer Registry for providing the data.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Christensen KG, Fenger-Gron M, Flarup KR, et al. Use of general practice, diagnostic investigations and hospital services before and after cancer diagnosis: a population-based nationwide registry study of 127,000 incident adult cancer patients. BMC Health Serv Res 2012;12:224.
- Innos K, Baburin A, Aareleid T. Cancer patient survival in Estonia 1995-2009: time trends and data quality. Cancer Epidemiol 2014;38:253–8.
- Sant M, Aareleid T, Berrino F, et al. EUROCARE-3: survival of cancer patients diagnosed 1990-94-results and commentary. Ann Oncol 2003;14 Suppl 5:v61–118.
- Crocetti E, Mallone S, Robsahm TE, et al. Survival of patients with skin melanoma in Europe increases further: Results of the EUROCARE-5 study. Eur J Cancer 2015;51:2179–90.
- Innos K, Padrik P, Valvere V, et al. Sex differences in cancer survival in Estonia: a population-based study. BMC Cancer 2015;15:72.
- Balch CM, Soong SJ, Atkins MB, et al. An evidence-based staging system for cutaneous melanoma. CA Cancer J Clin 2004;54:131–49.
- Homsi J, Kashani-Sabet M, Messina JL, et al. Cutaneous melanoma: prognostic factors. Cancer Control 2005;12:223–9.
- Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 2009;27:6199–206.
- Ederer F, Heise H, Instructions to IBM 650 programmers in processing survival computations. Methodological note no. 10. Bethesda: End Results Evaluation Section, National Cancer Institute; 1959.
- Brenner H, Rachet B. Hybrid analysis for up-to-date long-term survival rates in cancer registries with delayed recording of incident cases. Eur J Cancer 2004;40:2494–501.
- Corazziari I, Quinn M, Capocaccia R. Standard cancer patient population for age standardising survival ratios. Eur J Cancer 2004;40:2307–16.
- Dickman PW, Coviello E. Estimating and modeling relative survival. Stata J 2015;15:186–215.
- Godar DE. Worldwide increasing incidences of cutaneous malignant melanoma. J Skin Cancer 2011;2011:858425.
- Wallingford SC, Alston RD, Birch JM, et al. Increases in invasive melanoma in England, 1979-2006, by anatomical site. Br J Dermatol 2011;165:859–64.
- Leiter U, Garbe C. Epidemiology of melanoma and nonmelanoma skin cancer-the role of sunlight. Adv Exp Med Biol 2008;624:89–103.
- Thompson JF, Scolyer RA, Kefford RF. Cutaneous melanoma. Lancet 2005;365:687–701.
- Tekkel M, Veideman T, Health behavior among Estonian adult population, 2014. Tallinn: National Institute for Health Development; 2015.
- Engholm G, Ferlay J, Christensen N, et al. NORDCAN: Cancer Incidence, Mortality, Prevalence and Survival in the Nordic Countries, Version 7.2 (16.12.2015). Association of the Nordic Cancer Registries. Danish Cancer Society; 2016. Available from: http://www.ancr.nu/ [last accessed on 02/05/2016: Danish Cancer Society].
- Buettner PG, Leiter U, Eigentler TK, et al. Development of prognostic factors and survival in cutaneous melanoma over 25 years: an analysis of the Central Malignant Melanoma Registry of the German Dermatological Society. Cancer 2005;103:616–24.
- Bay C, Kejs AM, Storm HH, et al. Incidence and survival in patients with cutaneous melanoma by morphology, anatomical site and TNM stage: a Danish Population-based Register Study 1989-2011. Cancer Epidemiol 2015;39:1–7.
- Lyth J, Eriksson H, Hansson J, et al. Trends in cutaneous malignant melanoma in Sweden 1997-2011: thinner tumours and improved survival among men. Br J Dermatol 2015;172:700–6.
- De Angelis R, Sant M, Coleman MP, et al. Cancer survival in Europe 1999-2007 by country and age: results of EUROCARE-5-a population-based study. Lancet Oncol 2014;15:23–34.
- Crocetti E, Fancelli L, Manneschi G, et al. Melanoma survival: sex does matter, but we do not know how. Eur J Cancer Prev 2016;25:404–9.
- Nosrati A, Wei ML. Sex disparities in melanoma outcomes: the role of biology. Arch Biochem Biophys 2014;563:42–50.
- Kelly JW. Melanoma in the elderly – a neglected public health challenge. Med J Aust 1998;169:403–4.
- Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol 2001;19:3622–34.
