Abstract
Background: We report long-term outcomes of patients treated with primary radiotherapy (RT) or surgery and adjuvant RT for salivary gland malignancies.
Materials and methods: From 1964 to 2012, 291 patients received primary RT (n = 67) or RT combined with surgery (n = 224).
Results: The 5-, 10-, and 15-year local control, local-regional control, distant metastasis-free survival, cause-specific survival and overall survival rates were 82%, 77% and 73%; 77%, 72% and 67%; 74%, 70% and 70%; 70%, 59% and 54%; and 63%, 47% and 38%, respectively. Per multivariate analysis, combined surgery and RT and T stage impacted local control; overall stage and combined surgery and RT impacted local-regional control; overall stage impacted distant metastasis-free survival; and overall stage, node positivity, clinical nerve invasion, and surgery and RT impacted cause-specific and overall survival. Five percent of patients experienced grade 3 or worse toxicity.
Conclusion: Combined surgery and RT improves local control, local-regional control, and cause-specific survival compared with primary RT for salivary tumors.
Salivary gland malignancies comprise a rare and heterogeneous subset of head and neck carcinomas. Representing less than 5% of all new cancer diagnoses, multi-institutional, prospective studies are a challenge to perform. Standard of care, based primarily on single-institution retrospective series, involves surgery at the primary site followed by adjuvant radiotherapy (RT) for high-risk features such as high-grade histology or low-grade tumors that are multiply recurrent or have positive margins [Citation1–9]. Although clinically or radiographically positive neck disease should undergo therapeutic neck dissection, the role for elective neck dissection remains limited as it is not indicated in low-grade malignancies; elective neck irradiation provides excellent control rates for high-grade or large (T3 or T4) tumors [Citation10]. The indications for RT alone remain restricted to patients for whom medical comorbidity excludes surgery, surgical excision would be too morbid, or gross total resection is unachievable. With the goal of further refining the management of this heterogeneous disease, herein we present the outcomes for patients treated for salivary gland carcinomas at a high volume center with over 40 years of potential follow-up.
Material and methods
Under an institutional review board approved protocol, we reviewed the medical records of all patients with previously untreated salivary gland carcinomas treated with curative intent between October 1964 and September 2012 at the University of Florida using RT alone (n = 67) or RT combined with surgery (n = 224). This is an update of a prior study published in 2005 [Citation11]. Patients with metastatic disease on presentation, squamous cell carcinoma histology, tumors arising from the lacrimal gland, trachea, cervical esophagus and an unknown primary site were excluded.
Patient, tumor and treatment characteristics are shown in . The median patient age in our cohort was 58 years (range 11–89 years). The median follow-up for all patients was seven years (range <1–43 years). The median follow-up for living patients was 12 years (range 1–43 years). At 5, 10 and 15 years, 32, 21 and 16 of 67 patients were alive after RT alone and 139, 82 and 50 of 224 patients were alive after combined RT and surgery, respectively. All patients were clinically staged according to the seventh edition of the American Joint Committee on Cancer (AJCC) staging system [Citation12]. Those with completely resectable tumors underwent surgery and received postoperative RT for close or positive margins, tumors involving regional lymph nodes, high-grade histology, perineural invasion and/or lymphvascular space invasion. RT alone was reserved for patients with advanced-stage, incompletely resectable tumors or those with early-stage lesions whose surgical options were limited by comorbid conditions or for whom the functional deficit associated with resection was believed to be suboptimal. A few patients with borderline resectable tumors underwent preoperative RT. Nine patients received adjuvant chemotherapy as part of their initial treatment.
Table 1. Patient, tumor and treatment characteristics.
RT was administered once daily to 160 patients, twice daily to 122 patients and nine patients received a concomitant boost. The median dose delivered was 74 Gy (range 50–79.2 Gy) for RT alone; 70 Gy (range 55–85 Gy) for postoperative RT; and 60 Gy (range 45–64.8 Gy) for preoperative RT. All patients received photon and/or electron RT; no patient was treated with protons, neutrons or carbon therapy. A brachytherapy boost was combined with external-beam RT in nine patients. Forty-seven patients received intensity-modulated radiotherapy (IMRT).
Surgery as part of the initial treatment consisted of resection of the primary tumor in 148 patients, resection of the primary cancer combined with neck dissection in 76 patients and neck dissection alone in four patients. Patients who received neck dissection combined with RT alone to the primary site are included in the RT-alone group for analyses. Twenty-six patients underwent neck dissection and found to have pathologically N0 necks: 58% (n = 15) had high-grade salivary gland cancers, 12% (n = 3) had low-grade major salivary malignancies, 27% (n = 7) had high-grade minor salivary gland tumors and 4% (n = 1) had low-grade minor salivary gland malignancies. Adenoid cystic carcinomas were considered to be high grade. One of the four patients with low-grade histology who underwent elective neck dissection had a low-grade mucoepidermoid tumor and the other three had acinic cell carcinomas. The median interval between surgery and RT was 36 days (range 16–189 days). Resection margins were negative in 79 patients, microscopically positive in 131 patients, and macroscopic residual tumor was left in 14 patients. Close margins (<5 mm) were not included in the group of patients with positive margins.
The neck was clinically negative at diagnosis in 224 patients. The decision whether to observe or electively treat the neck depended on the primary site, histology and the attending physician. An N0 neck was observed in 48 patients, electively dissected in 27 patients and electively irradiated in 149 patients. The postoperative dose to the dissected positive neck was 60–70 Gy. The undissected clinically node-negative neck received 50–60 Gy, the latter to the upper neck depending on primary site. Complications were coded according to the Common Terminology Criteria for Adverse Events (CTCAE), version 4.0 [Citation13].
The Kaplan-Meier product-limit method was used to calculate the rates of local control, local-regional control, distant metastasis-free survival, cause-specific survival and overall survival. A Cox proportional hazards regression analysis of various endpoints was performed using eight explanatory variables. Explanatory variables included in the multivariate analyses were as follows: sex, site (major vs. minor), tumor (T) classification (T1-T3 vs. T4), nodal (N) classification (N0 vs. N positive), overall stage (I-III vs. IV), perineural invasion (none or incidental vs. clinical) and treatment group (surgery and adjuvant RT vs. RT alone). Clinical perineural invasion defined paresis of a named nerve at presentation, and not enough patients received adjuvant chemotherapy to include it in the analyses.
Results
Time to disease recurrence
The median time to disease recurrence after treatment for all patients was 1.6 years (range <1–20 years). Within 5, 10 and 15 years of treatment, 85%, 95% and 98% of treatment failures were observed, respectively. Of the patients treated with macroscopic residual disease there was only one (7%) isolated local recurrence. One (7%) other patient had a local and regional failure and one (7%) had an isolated regional failure. Of those with microscopic residual disease, isolated local or regional recurrences occurred in 8% (n = 10) and 5% (n = 6), respectively. Of those with gross total resection, isolated local or regional recurrences were observed in 5% (n = 4) and 1% (n = 1), respectively. Of those with a local-regional recurrence, eight underwent salvage surgery and four were successfully salvaged. Of those with regional recurrences, seven underwent salvage surgery and two were successfully salvaged.
Disease control
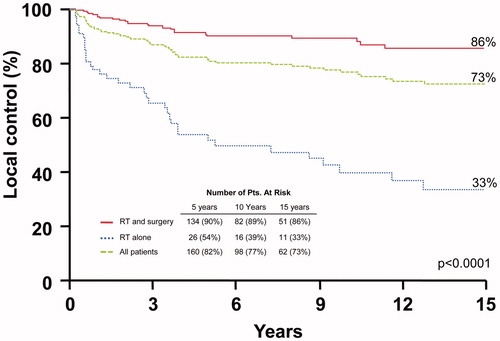
The 10-year local control rates after surgery and adjuvant RT compared with RT alone were: T1-T3, 93.6% versus 76.3% (p = .0020); and T4, 72.0% versus 17.3% (p < .0001). shows the rates of local control. Multivariate analysis showed that T stage and addition of surgery predicted for local control. Hazard ratios were 0.2 (range 0.1–0.4; p ≤ .0001) and 4.3 (range 2.5–7.5; p ≤ .0001), respectively.
Figure 1. Local control rates after surgery and adjuvant radiotherapy (RT), RT alone, and for the overall group.
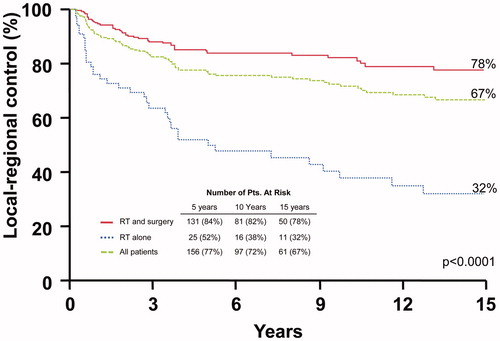
shows the rates of local-regional control. The 10-year local-regional control rates after surgery and RT compared with RT alone were: stages I-III, 88.8% versus 71.5% (p = .0195); and stage IV, 68.5% versus 20.4% (p < .0001). Multivariate analysis revealed that both overall stage and the addition of surgery predicted for local-regional control. Hazard ratios were 3.6, (2.1–6.0; p ≤ .0001) and 3.1 (1.9–5.0; p ≤ .0001), respectively. There were only four (3%) isolated nodal recurrences in cN0 patients who received elective nodal irradiation.
Figure 2. Local-regional control rates after surgery and adjuvant radiotherapy (RT), RT alone, and for the overall group.
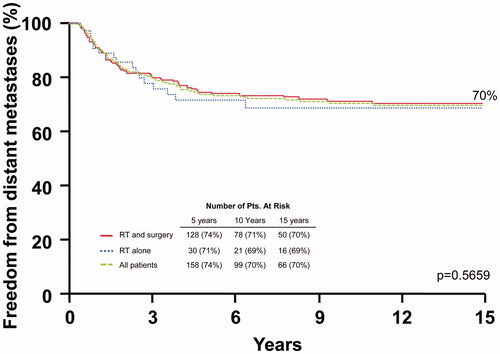
shows the rates of distant metastasis-free control. The 10-year distant metastasis-free survival rates after surgery and RT versus RT alone were: stage I-III, 78.7% versus 94.1% (p = .2550); and stage IV, 56.8% versus 55.2% (p = .8514). Multivariate analysis revealed that only overall stage impacted this endpoint with a hazard ratio of 2.9 (1.8–4.5; p ≤ .0001).
Figure 3. Distant metastasis-free survival rates after surgery and adjuvant radiotherapy (RT), RT alone, and for the overall group.
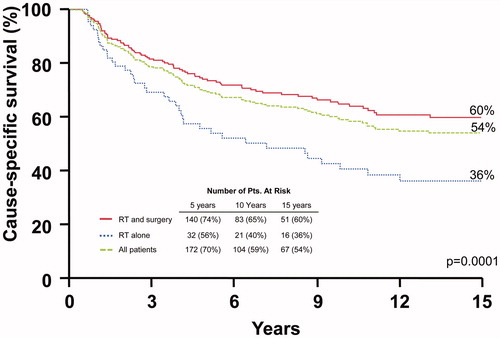
depicts cause-specific survival rates. The 10-year cause-specific survival rates after surgery and RT compared with RT alone were: I-III, 78.4% versus 92.9% (p = .3339); and IV, 42.0% versus 20.6% (p = .0366). Multivariate analysis showed that overall stage, nodal involvement, clinical nerve involvement and the addition of surgery significantly impacted this endpoint. Hazard ratios were 3.3 (1.6–7.1; p ≤ .0001), 1.7 (1.0–3.0; p < .05), 1.7 (1.0–2.8; p < .05), and 1.4 (0.9–2.2; p < .05), respectively.
Figure 4. Cause-specific survival rates after surgery and adjuvant radiotherapy (RT), RT alone, and for the overall group.
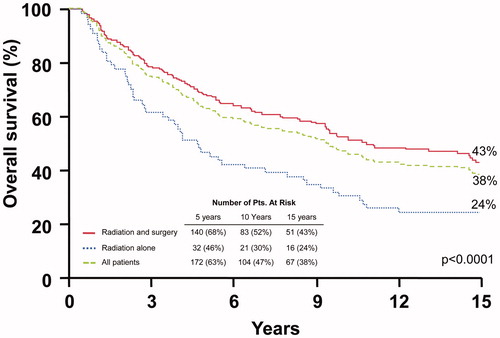
shows the overall survival rates. The 10-year overall survival rates after surgery and RT compared with RT alone were: I-III, 64.9% versus 63.2% (p = .4074); and IV, 30.3% versus 16.7% (p = .0999). Multivariate analysis revealed that overall stage, nodal involvement, clinical nerve involvement and the addition of surgery significantly impacted this endpoint. Hazard ratios were 2.1 (1.5–3.0; p < .0001), 1.8 (1.3–2.6; p < .01), 1.7 (1.2–2.4; p < .01) and 1.6 (1.1–2.2; p < .01), respectively.
Complications
Eight patients (4%) experienced an acute surgical complication. There was one grade 5 postoperative meningitis following a salvage resection and seven patients who required additional surgical interventions for either necrosis (n = 2), bleeding (n = 2), fistula (n = 1), fracture (n = 1), or deformity (n = 1). Long-term radiation complications included two secondary tumors, one atypical meningioma and one benign olfactory meningioma within the treatment fields diagnosed 24 and 26 years following RT, respectively. Grade 3 long-term toxicities included 14 patients (5%) with osteoradionecrosis following RT with a median latency of eight years (range <1–20 years). Two patients were gastrostomy tube-dependent. One of 47 patients treated with IMRT experienced grade 3 toxicity. Seventeen patients (6%) had anticipated side effects including vision loss (n = 12) or hearing loss (n = 5). Only one of these patients was treated with IMRT. There were slightly fewer grade 3 toxicities associated with IMRT (4%; n = 2) as aforementioned although this may be in part related to the shorter duration of follow-up in this cohort.
Discussion
The role of adjuvant RT
Although low-grade salivary carcinomas can be treated with surgery alone, the standard of care for salivary neoplasms with high-risk features remains adjuvant RT [Citation14]. Although the role of adjuvant RT in the treatment of salivary carcinomas has not been studied in a randomized, prospective cohort, several retrospective studies have shown increased local and local-regional control with adjuvant RT across various histologic subtypes and head and neck subsites [Citation1–9].
Terhaard et al. reviewed patients with mostly major salivary gland tumors treated with surgery alone or surgery and postoperative RT [Citation15]. The addition of postoperative RT improved 10-year local control rates significantly compared with surgery alone in patients with T3-4 tumors (84% vs. 18%), those with close margins (95% vs. 55%) and incomplete resections (82% vs. 44%), those with bone invasion (86% vs. 54%) and those with perineural invasion (88% vs. 60%). Our multivariate analysis corroborates these results in that stage T4 significantly adversely impacted local control, and clinical perineural invasion adversely impacted overall survival. Garden et al. published a report on 160 patients and also found that postoperative RT improved local control in patients with minor salivary gland tumors [Citation16].
Our report includes a large number of patients with adenoid cystic carcinomas (37%). These tumors tend to have an indolent growth rate and a low probability of regional lymph node metastases, but a high risk of hematogenous dissemination. Chen et al. published a review of 140 patients with adenoid cystic carcinomas of the head and neck treated with either primary surgery or surgery and adjuvant RT. Of these patients, 64% received postoperative RT to a median dose of 64 Gy. Among those with T4 disease, perineural invasion and major nerve involvement, omission of postoperative RT was an independent predictor of local recurrence [Citation17]. The importance of adjuvant RT for adenoid cystic carcinomas is corroborated by earlier reports from the University of Florida and the MD Anderson Cancer Center (Houston, TX) which showed that positive margins, perineural invasion and major nerve involvement were adverse prognostic factors [Citation1,Citation2].
Neck management
Although the role for elective neck dissection remains limited in salivary malignancies, elective neck irradiation provides excellent control for those at risk for regional disease. Patients with small or low-grade tumors have a low risk for occult disease as evidenced by a report by Armstrong et al. on almost 500 patients who underwent elective neck dissection for salivary malignancies. Among the 407 node-negative patients, 47 (12%) were found to have nodal disease on elective node dissection. Multivariate analysis revealed that only tumor size and grade were significant risk factors. More specifically, tumors 4 cm or greater in size had a 20% (32 of 164 tumors) risk of occult metastases and high-grade lesions (regardless of histologic type) had a 49% risk [Citation18]. Although patients with clinically or radiographically positive neck disease should undergo a therapeutic neck dissection, those with a clinically negative neck who are at high risk for regional disease achieve high rates of control with elective neck irradiation. These findings are corroborated in a report by Herman et al. who analyzed 59 patients with high-grade salivary neoplasms and a clinically N0 neck who were treated with either elective neck dissection (n = 41) or elective neck irradiation (n = 18). At five years, the neck control rate for was 100% in the patients who received elective nodal irradiation. In our study, the control rate was 97% among the patients who received postoperative RT to the clinically N0 neck after elective nodal irradiation.
RT alone
Disease control with primary RT is inferior to disease control with combined surgery and RT. In 2006, Chen et al. reported on 45 patients treated to a median dose of 66 Gy (range 57–74 Gy) [Citation7]. With a median follow-up of 101 months (range 3–285 months), the 10-year local control and overall survival rates were 57% and 46%, respectively. These findings are consistent with our results which show a 10-year local control rate of 39% that declined to 33% at 15 years. As a result of the inferior local control with low-linear energy transfer (LET) RT alone, investigators have been studying the use of high-LET particles or proton therapy since the 1970s.
High-LET particles and proton therapy
While early investigations into neutrons have shown a benefit over photons in local-regional disease control, the benefit has not translated into longer survival. Laramore et al. published multiple series on the use of neutrons for inoperable primary or recurrent major or minor salivary gland tumors. The 10-year local-regional control probability was 17% after photon therapy and 56% after neutron therapy. This local-regional benefit did not translate into a survival benefit as many of the patients in the neutron cohort developed distant metastases and/or severe and life-threatening complications [Citation19]. Our study shows that 30% of patients will have distant metastasis at 15 years after photon RT, and only overall stage significantly impacted this endpoint.
More recently, investigators have analyzed both protons and carbon ions as a single-modality treatment for salivary gland tumors. Takagi et al. published their experience treating 80 patients with either protons (50%) or carbon ions (50%) to a median dose of 65 Gy over 26 treatments [Citation20]. With a median follow-up of almost three years, they reported five-year overall survival, progression-free survival, and local control rates of 63%, 39% and 75%, respectively. There was no discernable difference in outcomes between those treated with proton or carbon therapy although over a quarter of the patients experienced grade 3 or higher late toxicities, including three patients who developed grade 5 toxicities. Reports of small series of patients treated with carbon ions alone reveal higher control rates with lower toxicity compared with photon therapy, yet the data are still too limited draw conclusions [Citation21]. Much like the early neutron data, particle therapy for unresectable salivary tumors may improve local control, but the benefits in overall survival have not been shown and there may be an increased risk for clinically significant toxicity, which will be better determined with longer follow-up.
Our study is limited by the typical limitations of a single-institutional retrospective investigation of a heterogeneous population. A potential confounding factor when comparing RT alone versus surgery and RT is that the former group may have contained a small subset of patients who were not operated due to medical co-morbidities.
Treatment recommendations and advances in salivary gland tumors
Primary surgical resection is the first step in all patients who have lesions amenable to gross total resection and who are medically operable. There is currently no known role for chemotherapy. Our indications for treatment of low-grade or benign salivary tumors with positive, not close, margins or multiply recurrent disease is based on physician’s judgment and the number of recurrences and resection details. We do not treat the neck electively in low-grade or benign tumors. All high-grade malignant salivary tumors are treated with postoperative RT to the primary site and the undissected cN0 neck. Our bias is to treat high-grade cN0 neck disease with RT as well. We treat positive/close margins and nodal stations with extracapsular extension to 70 Gy, negative margins and pathologic node-positive stations to 60–66 Gy and the standard risk area to 60 Gy. Advances in the treatment of salivary gland tumors are largely related to higher resolution axial imaging, surgical expertise and the proliferation of high-quality conformal image-guided RT. While follow-up is still premature, the dosimetric advantages of IMRT and heavy ion therapy are likely to lead to lower treatment morbidity.
Combined surgery and RT leads to a significant increase in local and local-regional control and cause-specific survival over RT alone for salivary malignancies. There is no difference in the freedom from distant failure between surgery with RT and RT alone, suggesting a role for advances in systemic therapy. Although RT alone yields a cause-specific survival rate of 40% at 10 years, it should be reserved only for patients who are unsuitable for surgery or for whom an operation would be too morbid given the overall survival benefit with combined surgery and RT.
Disclosure statement
The authors report no conflicts of interest.
References
- Mendenhall WM, Morris CG, Amdur RJ, et al. Radiotherapy alone or combined with surgery for adenoid cystic carcinoma of the head and neck. Head Neck. 2004;26:154–162.
- Garden AS, Weber RS, Morrison WH, et al. The influence of positive margins and nerve invasion in adenoid cystic carcinoma of the head and neck treated with surgery and radiation. Int J Radiat Oncol Biol Phys. 1995;32:619–626.
- Pedersen D, Overgaard J, Sogaard H, et al. Malignant parotid tumors in 110 consecutive patients: treatment results and prognosis. Laryngoscope. 1992;102:1064–1069.
- Chen AM, Garcia J, Bucci MK, et al. The role of postoperative radiation therapy in carcinoma ex pleomorphic adenoma of the parotid gland. Int J Radiat Oncol Biol Phys. 2007;67:138–143.
- Rinaldo A, Shaha AR, Pellitteri PK, et al. Management of malignant sublingual salivary gland tumors. Oral Oncol. 2004;40:2–5.
- Storey MR, Garden AS, Morrison WH, et al. Postoperative radiotherapy for malignant tumors of the submandibular gland. Int J Radiat Oncol Biol Phys. 2001;51:952–958.
- Chen AM, Bucci MK, Quivey JM, et al. Long-term outcome of patients treated by radiation therapy alone for salivary gland carcinomas. Int J Radiat Oncol Biol Phys. 2006;66:1044–1050.
- Lopes MA, Santos GC, Kowalski LP. Multivariate survival analysis of 128 cases of oral cavity minor salivary gland carcinomas. Head Neck. 1998;20:699–706.
- Parsons JT, Mendenhall WM, Stringer SP, et al. Management of minor salivary gland carcinomas. Int J Radiat Oncol Biol Phys. 1996;35:443–454.
- Herman MP, Werning JW, Morris CG, et al. Elective neck management for high-grade salivary gland carcinoma. Am J Otolaryngol. 2013;34:205–208.
- Mendenhall WM, Morris CG, Amdur RJ, et al. Radiotherapy alone or combined with surgery for salivary gland carcinoma. Cancer. 2005;103:2544–2550.
- Edge S, Byrd DR, Compton CC, et al. American Joint Committee on Cancer (AJCC) Cancer Staging Manual. 7th ed. Chicago, IL: Springer; 2011.
- US Department of Health and Human Services, National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. 2009. Available from: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
- Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2,807 patients. Head Neck Surg. 1986;8:177–184.
- Terhaard CH, Lubsen H, Rasch CR, et al. The role of radiotherapy in the treatment of malignant salivary gland tumors. Int J Radiat Oncol Biol Phys. 2005;61:103–111.
- Garden AS, Weber RS, Ang KK, et al. Postoperative radiation therapy for malignant tumors of minor salivary glands. Outcome and patterns of failure. Cancer. 1994;73:2563–2569.
- Chen AM, Bucci MK, Weinberg V, et al. Adenoid cystic carcinoma of the head and neck treated by surgery with or without postoperative radiation therapy: prognostic features of recurrence. Int J Radiat Oncol Biol Phys. 2006;66:152–159.
- Armstrong JG, Harrison LB, Thaler HT, et al. The indications for elective treatment of the neck in cancer of the major salivary glands. Cancer. 1992;69:615–619.
- Laramore GE, Krall JM, Griffin TW, et al. Neutron versus photon irradiation for unresectable salivary gland tumors: final report of an RTOG-MRC randomized clinical trial. Radiation Therapy Oncology Group. Medical Research Council. Int J Radiat Oncol Biol Phys. 1993;27:235–240.
- Takagi M, Demizu Y, Hashimoto N, et al. Treatment outcomes of particle radiotherapy using protons or carbon ions as a single-modality therapy for adenoid cystic carcinoma of the head and neck. Radiother Oncol. 2014;113:364–370.
- Mizoe JE, Tsujii H, Kamada T, et al. Dose escalation study of carbon ion radiotherapy for locally advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2004;60:358–364.