Abstract
Background: Checkpoint inhibitors have proven effectiveness in clinical trials for non-small cell lung cancer (NSCLC) patients, but if this is congruent with routine patient care is discussed. We present real-world experience with the PD1-inhibitor nivolumab in NSCLC.
Patients and methods: Patients with NSCLC were considered eligible for nivolumab treatment after one or more lines of chemotherapy, and when in reasonable performance status (PS) [Eastern Cooperative Oncology Group (ECOG) < 3]. Treatment was given according to guidelines in the two phase III studies, CA209017 and CA209057. Response evaluation was done according to Recist 1.1, and treatment given until unequivocal progression or intolerable toxicity.
Results: Fifty-eight patients (30 females) commenced therapy in the period June–August 2015. Median age was 64.6 years (range 32.3–88.2). Twenty-four patients had squamous cell carcinoma and 32 adenocarcinoma, 38 had received two or more prior lines of therapy. Fourteen cases (24%) were in ECOG PS 2. After a medium observation time of 14.3 months, 13 (22%) are still in treatment. Median time to treatment failure (TTF) was 4.0 months, 34% were off treatment during the first two months. Median overall survival (OS) is 11.7 months. There was no difference in TTF or OS among patients with squamous versus non-squamous histology or between 1 versus >1 prior line of therapy. Four patients (7%) were off treatment due to toxicity, none were grade 4 or 5.
Conclusion: Nivolumab treatment outside clinical trials seems to perform as expected.
Metastatic non-small cell lung cancer (NSCLC) is invariably a deadly disease, but lately immunotherapy has shown durable responses in subsets of patients [Citation1–4]. So far, immunotherapy experience in lung cancer is mostly based on clinical trials, with selected patient cohorts. Real-world data on this therapy are awaited, as it is of importance to evaluate the generalizability of the trial data to the general lung cancer population [Citation5]. Herein, we report our experience with nivolumab treatment in an unselected cohort of 58 metastatic NSCLC patients treated in a named patient use program at the Norwegian Radium Hospital, Oslo, Norway.
Patients and methods
From June through August 2015, 58 metastatic NSCLC patients were referred to our tertiary cancer clinic from local hospitals, with the request of immunotherapy treatment, and all were reviewed and found eligible for treatment with nivolumab in a named patient program. We accepted Eastern Cooperative Oncology Group (ECOG) 0-2, all ages and all NSCLC histologies. PD-L1 status was not assessed. Treatment was given as in the phase III trials CA209017 and CA209058 (briefly, 3 mg/kg, every two weeks), and computed tomography (CT)-based evaluation performed every 6–8 weeks. All patients were prospectively followed until data cutoff 7 October 2016.
Statistical analyses were performed using GraphPad Prism v 7.00. As this is regarded a quality assurance study, no ethical committee permission is required.
Results
All patients commenced therapy in the period June–August 2015. Clinical characteristics are detailed in . No patients had known brain metastases. None of the adenocarcinomas were found to have ALK- or EGFR-aberrations except one patient with an insertion in EGFR exon 20. All patients had received at least one platinum-based regimen in metastatic setting. Twenty-two patients (38%) were initially diagnosed in stage I-III and later progressed. Time from initial diagnosis until first cycle of nivolumab varied from 4.5 to 99.9 months, with a median of 15.6 months. Four of the patients deteriorated from decision of treatment until the day of their first cycle, and was in ECOG PS 3 when starting therapy.
Table 1. Patient characteristics treated by nivolumab (n = 58).
A total of 678 nivolumab infusions were provided until data cutoff, and median number of cycles was 8.5 (range 1–32). Median number of days per cycle was 16.
After a median observation time of 14.3 months, 13 (22%) are still in treatment (). Median time to treatment failure (TTF) was 4.0 months, with 34.4% off treatment during the first two months. Median overall survival (OS) is 11.7 months (), and 27 patients (46.6%) are alive at time of data cutoff. There was no difference in TTF or OS among patients with squamous versus non-squamous histology or between 1 versus >1 prior line of therapy ( and Supplementary Fig 1). Time from diagnosis to start of nivolumab was not correlated to treatment benefit (). One patient developed brain metastases during therapy.
Figure 1. Time to treatment failure (a) and overall survival (b) for patients (n = 58) treated with nivolumab in named patient program.
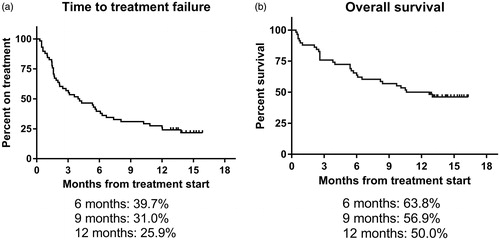
Figure 2. Overall survival for patients with non-squamous carcinoma (n = 34, solid line) or squamous cell carcinoma (n = 24, dotted line).
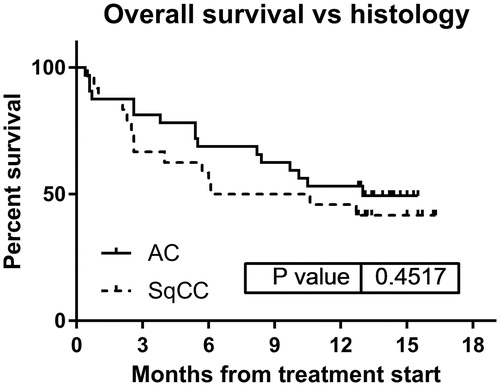
Figure 3. Time (months) from diagnosis to nivolumab treatment start (white), time on nivolumab treatment (black) and post-nivolumab survival time (grey). Each bar represents one patient. Patients still alive are marked with arrow.
Fifteen patients survived six months or more after nivolumab discontinuation without further therapy, 10 of these are still alive at data cutoff ().
Four patients (6.9%) were off treatment due to toxicity, none were grade 4 or 5. Drug-related adverse events were reported in 18 patients (31%). Typical toxicities included diarrhea, radiographic signs of pneumonitis and pruritus, as well as one patient with pituitary dysfunction needing mineralocorticoid supplements ().
Table 2. Adverse events during nivolumab therapy (n = 58).
Discussion
In a fairly large unselected cohort of nivolumab-treated NSCLC patients, we find response rates, TTF, OS rates as well as toxicity frequencies which compare favorably with published data from phase III trials. The one-year survival rate in the two phase III trials was 42% for squamous cell carcinoma group [Citation2] and 51% for the non-squamous group [Citation1], whereas in our dataset 50% are still alive at 12 months (45.8% for squamous cell carcinomas, and 53.1% for non-squamous). The favorable results remain also in patients with reduced performance status.
It is interesting to note the lack of correlation with time from initial diagnosis as well as number of previous treatment lines, and nivolumab response. Whether previous chemotherapy treatment may induce higher response rates in NSCLC as seen in ovarian cancer [Citation6] is unknown. Indeed, higher responses of immunotherapy in first line versus later lines are reported in lung cancer [Citation7].
The fact that a substantial fraction of patients survive for >6 months after discontinuation, without receiving further therapy is intriguing, and seems to be specific for immunotherapy. Such long posttreatment survival is not regularly seen in second-line chemotherapy in NSCLC [Citation8].
In summary, our findings from routine practice confirm the results and side effect profile from clinical trials, and show that immunotherapy may yield similar outcomes regardless of number of previous treatment lines, and result in similar responses also in ECOG PS 2 patients.
IONC_1253865_supplemental_figures.zip
Download Zip (172.9 KB)IONC_1253865_supplementary_figures.zip
Download Zip (232.9 KB)Acknowledgements
The collaboration with physicians at referring hospitals is highly appreciated. We thank Ingrid Lydersen for expert nursing assistance, and Ali Areffard, PhD, Scientific Advisor, Immuno-Oncology Bristol Myers-Squibb (BMS) Norway, for valuable support. Free drug for the first 475 cycles was provided by BMS, but no funding was provided for the execution of the treatment, or for writing or other aspects of this work.
Disclosure statement
The authors have declared no conflicts of interest.
Funding
The writing phase of this manuscript was partially funded by the Norwegian Cancer Society and the South-Eastern Norway Regional Health Authority.
References
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639.
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135.
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550.
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837–1846.
- Saturni S, Bellini F, Braido F, et al. Randomized controlled trials and real life studies. approaches and methodologies: a clinical point of view. Pulm Pharmacol Ther. 2014;27:129–138.
- Böhm S, Montfort A, Pearce OM, et al. Neoadjuvant chemotherapy modulates the immune microenvironment in metastases of tubo-ovarian high-grade serous carcinoma. Clin Cancer Res. 2016;22:3025–3036.
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028.
- Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–1597.
