Abstract
Background: The primary objective of this study was to compare the progression-free survival (PFS) at 12 weeks between patients treated with IGF-1R pathway modulator AXL1717 (AXL) and patients treated with docetaxel (DCT).
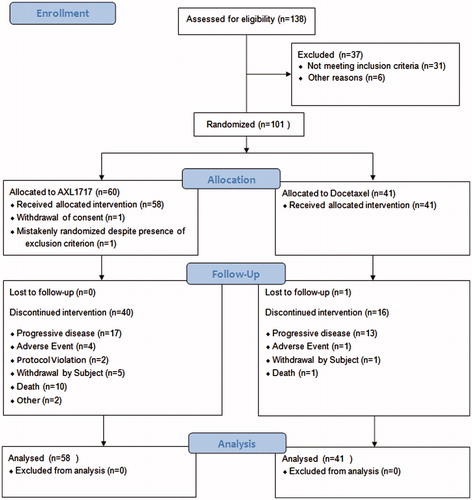
Material and methods: The study was conducted at 19 study centers in five countries. A total of 99 patients with previously treated, locally advanced or metastatic non-small cell lung cancer (NSCLC) of the squamous cell carcinoma (SCC) or adenocarcinoma (AC) subtypes in need of additional treatment were randomized and treated with either 300 or 400 mg of AXL as daily BID treatment (58 patients) or DCT given as 75 mg/m2 in three-week cycles (41 patients) as monotherapy in a 3:2 ratio for each NSCLC subtype. Patients were treated in the primary study treatment period for a maximum of four treatment cycles.
Results: The 12-week PFS rate, median PFS and overall survival (OS), as well Kaplan-Meier hazard ratio for PFS and OS, did not show any statistically significant differences between the treatment groups. For the primary endpoint, the AXL group had a lower percentage of patients (25.9%) who were progression-free at Week 12 as compared to the DCT group (39.0%), although the difference was not statistically significant. The most notable difference in the incidence of treatment emergent adverse effects (TEAEs) was the lower incidence of treatment-related grade 3/4 neutropenia in patients treated with AXL.
Conclusion: These results suggest neither of the treatments to be superior of the other when treating locally advanced or metastatic NSCLC. Considering the lower incidence of grade 3/4 neutropenia in the AXL group this treatment warrants further research.
Lung cancer is today the most common cancer and also the leading cause of cancer-related death in the western world [Citation1]. Patients with advanced disease who relapses after initial therapy are often given docetaxel (DCT) [Citation2]. The prognosis is dismal with five-year survival rates of <5% for these patients [Citation3].
The insulin-like growth factor 1 receptor (IGF-1R) signaling pathway is considered to be a valid target for anticancer pharmaceutical development. The relatively high levels of free IGF-1 in adenocarcinoma (AC) and squamous cell carcinoma of the lung (SCC) suggest these cancers to be the potentially promising areas for development of drugs that target the (IGF-1R) signaling pathway [Citation4–9].
AXL1717 (AXL) is an oral small-molecule pharmaceutical agent, originally developed as a targeted modulator of (IGF-1R) signaling [Citation10]. Its anti-tumoral effect is suggested to derive from indirect effects on microtubule dynamics leading to cell cycle arrest in mitosis and subsequent mitotic catastrophe. Based on the absence of direct binding to beta-tubulin and on the benign clinical side effect profile, the effect on microtubule seems to differ from microtubule inhibitors presently used clinically [Citation11,Citation12]. Several anti-IGF-1R antibodies have been tested clinically against a wide range of cancers, including lung cancer, with disappointing results [Citation13,Citation14]. The dual mechanism of AXL (IGF-1R modulation and cell cycle arrest) may therefore provide a mechanism for anti-tumoral activity of clinical interest.
In the present randomized phase II study, a total of 99 patients with previously treated, locally advanced or metastatic SCC or AC subtypes of non-small cell lung cancer (NSCLC) were randomized in 3:2 ratio for each NSCLC subtype, to either DCT as monotherapy or AXL as monotherapy with the primary aim of comparing the rate of progression-free survival (PFS) at 12 weeks.
Material and methods
The study was designed as an open-label, randomized, multicenter, phase II study to compare AXL to standard DCT as second-line treatment of SCC and AC of the lung. Also third-line treatment was allowed in the AC group. An overview of the study design and patient disposition is shown in . The study has been registered in ClinicalTrials.gov (NCT01561456).
Patients with histologically confirmed diagnosis of locally advanced, or metastatic (stage IIIB or IV) SCC or AC histologic subtype of NSCLC who had received one (SCC) or one or two (AC) previous lines of antitumor treatment were included. The first patient was included 19 December 2011 and the study was terminated 1 September 2013.
Based on results from clinical trials reported by Shepherd and Fossella [Citation15,Citation16], a 12-week PFS of 42.6% was expected with DCT in second-line NSCLC treatment. It was suggested by Regulatory Authorities to design this phase II study with 50% power for the assessment of the primary endpoint as the study was performed in a phase II setting. In the original protocol, an expected overall sample size of 140 provided 50% power to find a statistically significant difference at the 5% level (two-sided test), with 84 patients in the AXL group and 56 patients in the DCT group, if the PFS rate with AXL was 61.1%. An interim review of efficacy data in May 2013 showed that OS results were supportive of comparability of AXL to standard treatment with DCT, and as the probability for either treatment to show superiority over the other with respect to OS was regarded as low, the Sponsor curtailed enrollment in the study from a planned 140 treated patients to the 99 patients described in this report. At the end of the screening period, when a patient was determined to be eligible for enrollment, the site contacted the interactive web-based randomization system (IWRS) and the patient was assigned a patient number and was randomly allocated to either the AXL group or the DCT group. No blinding of study medications was performed.
Randomization with stratification (SCC and AC subtypes of NSCLC) was used to avoid bias in assigning patients to treatment, to increase the likelihood that known and unknown patient attributes (e.g. demographics and baseline characteristics) were evenly balanced across treatment groups, and to enhance the validity of statistical comparisons across treatment groups. Patients were to be randomized to either AXL or to DCT as monotherapy in a 3:2 ratio for each NSCLC subtype.
The study was subject to initial and continuing ethical review by an Institutional Review Board (IRB) and Independent Ethics Committee (IEC).
The study was conducted at 19 study centers: Belarus (1), Hungary (3), Poland (1), Russia (6), and Ukraine (8). Before initiation of the study at each investigative site, the protocol, the informed consent form(s), the patient information sheet, details of patient recruitment, and any other relevant study document were submitted to the responsible ethics committees and written approvals were obtained.
Patients were treated in the primary study treatment period for a maximum of four treatment cycles. A treatment cycle in the primary period was 21 days, unless dose delay or dose interruption was required. Patients treated with AXL, who responded to treatment or remained stable at the end of four cycles in the primary treatment period could be offered an extension of treatment with AXL. Such extension treatment was initiated by the Investigator and decided in consultation with the Sponsor on a case-by-case basis. Extension treatment cycles were 42 days with the same dose as in the primary study period (28 days of AXL treatment followed by a 14-day treatment-free interval), and the extension period could continue for up to four extension cycles (22 weeks) as long as the patient continued to benefit from AXL treatment.
AXL was provided in a ready-to-use suspension for oral administration. In the original protocol, AXL was administered as 400 mg given twice daily (BID) for the first 21 days of each cycle. Following safety concerns addressed in Protocol Amendment 2, AXL was administered as 300 mg for the first 28 days (21 days in Cycle 1 and the first seven days of Cycle 2). Then, depending on ANC levels measured during the first 28 days, subsequent doses could be increased to 400 mg BID, remain at 300 mg BID, or be temporarily interrupted and, when ANC levels recovered to an acceptable level, be resumed at the same dose or one dose level lower.
DCT was administered as a standard 75 mg/m2 in 100 ml of normal saline solution with intravenous (IV) infusion over 60 minutes on Day 1 of each 21-day treatment cycle for up to four cycles, according to local institutional procedures and standards. All patients receiving DCT were to be pre-medicated with corticosteroids, such as dexamethasone, according to local standard of care in order to reduce the incidence and severity of fluid retention as well as the severity of hypersensitivity reactions. Dose delays and adjustments were made for toxicities.
The primary objective of the study was to compare the rate of PFS at 12 weeks between patients treated with AXL and patients treated with DCT in the total study population and in the SCC and AC subtypes of NSCLC. PFS was defined as the time from randomization to the first observation of disease progression according to the RECIST criteria or death due to any cause. Patients were considered to be progression-free if they had a valid 12-week assessment by the central reviewer resulting in complete response (CR), partial response (PR) or stable disease (SD). Computed tomography (CT) scans performed between Days 56 and 113 were regarded as valid assessments for the primary endpoint. If no central assessment was available, a suitable local assessment was used instead.
Tumor assessment was performed at baseline and then at the end of Cycles 2 and 4. RECIST assessments of images related to the primary endpoint (baseline and the 12-week assessment) were repeated by a central independent radiologist as confirmation during the primary treatment period. During the extension period, RECIST assessments were conducted locally after Extension Cycles 2 and 4 (10 and 22 weeks) or at clinical signs of tumor progression at the discretion of the Investigator. Disease progression and survival status were obtained for all patients until death or the end of the study.
Safety evaluations included ongoing monitoring of adverse events (AEs), vital signs, physical examinations, clinical laboratory tests and electrocardiographies (ECGs). Safety during the conduct of the trial was monitored by the data safety monitoring committee (DSMC).
The DSMC was an independent, multidisciplinary advisory group responsible for the safety of the study patients. It was composed of senior biomedical and statistical experts with experience in the conduct of clinical studies, especially in studies for NSCLC. The DSMC provided recommendations about stopping or continuing the study, modifications of the AXL dose, or an alternate AXL treatment schedule, if needed.
All data were processed and summarized by the use of SAS® Version 9.0 or higher. All statistical tests were two-sided at the 0.05 level of significance.
Results
Antitumor efficacy
The patient baseline characteristics are shown in . The primary endpoint of the study was the PFS rate at 12 weeks as assessed by an independent blinded reviewer. PFS rate was defined as the proportion of surviving, non-progressing patients at 12 weeks (±4 weeks). Patients with no data for assessment of PFS at 12 weeks (early discontinued, death, tumor assessment was not done or not available, and other reasons) were classified as progressed (non-responders).
Table 1. Baseline characteristics.
For the primary endpoint, 15 patients (25.9%) the AXL group were progression-free at the 12-week assessment compared with 16 patients (39.0%) in the DCT group (p = 0.191). Of interest is that the disease progression at 12 weeks was roughly similar in the two groups with 13 patients (22.4%) in the AXL compared with seven patients in the DCT group (17.1%). Reasons for the differences included death prior to the 12-week assessment (20.7% compared with 12.2%, AXL vs. DCT) and assessments outside of the protocol window (13.8 vs. 7.3%). Six early fatal events in the AXL group were assessed as being related to AXL treatment as discussed under the safety section.
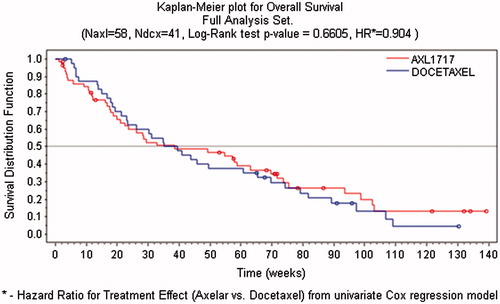
PFS was also estimated with the Kaplan-Meier procedure. These data are displayed in . The median overall survival (OS) was 38.7 in the AXL group compared with 37.4 weeks in the DCT group with a hazard ratio of 0.904 (p = .664). The OS data assessed by the Kaplan-Meier procedure showed similar OS for the AXL-treated patients in comparison to the DCT cohort for all patients as well as for the patients in the subgroups of AC and SCC.
RECIST tumor response assessed by a central independent blinded reader at 12 weeks showed that no patient had a CR in either treatment group. A total of five PRs were found by the central reader at the 12-week assessment in the DCT group compared with none in the AXL group (p = .010). The objective response rate (CR + PR) at 12 weeks by central reader was thus significantly lower (p = .010) for the AXL group (0.0%) compared to the DCT group (12.2%). The disease control rate (CR + PR + SD) by central reader was 24.1% for the AXL group and 36.6% for the DCT group (p = .189).
Safety
The grade 3/4 AEs were more common in the DCT group with 51% of the patients reporting at least one grade 3/4 adverse event compared with 41% in the AXL group (). The main side effects in both groups were bone marrow related with neutropenia (leukopenia) being the dominating in the adverse event profile. The laboratory data showed that 22% of the AXL-treated patients reported at least one occurrence of grade 3/4 neutropenia compared with 54% in the DCT group. Hyperglycemia was observed in one patient in the AXL group (1.7%) and in no patients in the DCT group.
Table 2. Summary of grade 3/4 treatment emergent adverse events and treatment-related adverse events overall and by preferred term occurring in two patients or more.
In the AXL treatment group, the serious treatment emergent adverse effects (TEAEs) that occurred in ≥5% of the patients were neutropenia (8.6%) and leukopenia (5.2%). All of the occurrences of these events were related to treatment. In the DCT treatment group, serious neutropenia occurred in 4.9% of the patients; no incidences of serious leukopenia occurred in the DCT treatment group. All of the occurrences of serious neutropenia in the DCT group were related to treatment.
Fatal events
A total of 17 deaths in the study were related to TEAEs. Of these, 12 occurred in the AXL group (21%) and five in the DCT group (12%). Disease progression was related to three deaths in the AXL group (5%) and four in the DCT group (10%), events with neutropenia were related to five deaths in the AXL group only (9%), and pulmonary bleeding was related to two deaths in the AXL group only (3%). Other reasons (respiratory failure; heart and respiratory failure) were reported for two patients in the AXL group (3%) and one patient (pulmonary edema) in the DCT group (2%).
The events with neutropenia that led to death for five patients in the AXL group were assessed as related or possibly related to study treatment. Of the remaining 12 deaths related to TEAEs, the fatal events were assessed as not related to study treatment.
Following the deaths of 12 patients in the study (nine in the AXL group and three in the DCT group), with seven patients dying within four weeks of entry into the study, enrollment was temporarily suspended while the DSMC convened three times and reviewed the cases and recommended an amendment to the protocol (7 October 2012). The deaths included four cases of neutropenia, of which three were not treated with antibiotics but were treated with corticosteroids, and two cases of pulmonary hemorrhage in patients with central squamous tumors with tumor involvement of major blood vessels on CT. In the amended protocol, it was therefore stated that SCC patients with radiologically verified involvement of the major vessels were to be excluded and the management of patients with neutropenia was described in a special document and guidelines with respect to prophylactic antibiotic treatment and the use of granulocyte colony-stimulating factors (G-CSF) in neutropenic patients were provided to all study centers. In the original protocol, the use of G-CSF was prohibited and led to withdrawal from the study. However, after protocol amendments, recommendations for treatment of neutropenic events with G-CSF were specified. At the time recruitment was suspended, 75 patients had been treated (46 AXL and 29 DCT). After the review by the DSMC, 23 patients (14 AXL and 9 DCT) already randomized continued receiving study treatment.
Discussion
In the present study, a total of 99 patients with previously treated, locally advanced or metastatic NSCLC were randomized to either AXL (58 patients) or DCT (41 patients). The results shows that median OS time was 38.7 weeks for the AXL group and 37.4 weeks for the DCT group (p = .661), thus no superiority of any the treatment groups could be shown.
In the second-line setting, DCT was the first drug approved clinical use based on the results of two phase III trials which proved DCT to be superior to best supportive care [Citation15] and to treatment with vinorelbine or ifosfamide [Citation16] in terms of OS. Other drugs that have proven to be effective in the second-line setting include pemetrexed (non-squamous NSCLC) [Citation17] and erlotinib [Citation18]. Recently, promising data has also emerged concerning the programed death 1 (PD-1) immune-checkpoint–inhibitor antibody nivolumab that has been shown to be superior to DCT in advanced NSCLC in the second-line setting [Citation19].
In a previous phase I/II study by Ekman et al., intermittent treatment with AXL for up to 28-days with 14–21-day treatment-free periods in between, was associated with RECIST-confirmed tumor response in one patient with squamous NSCLC [Citation11]. In the present study, RECIST assessment at 12 weeks showed that no patients had achieved a CR in either treatment group, whereas a total of five PRs were found in the DCT group compared with none in the AXL group. There was no statistically significant difference in the disease control rate (CR + PR + SD) between the AXL group and the DCT group.
A major drawback of the present study that has to be acknowledged is the lack of molecular characterization of the tumors. As a consequence, we have no data on IGF-1R expression in the tumors of the treated patients. As IGF-1R is the target of the study drug AXL, analysis and quantification of IGF-1R expression in relation to survival could have provided insight to whether IGF-1R expression in the tumor tissue could be used as a predictive factor for response to AXL treatment. In addition, IGF-1R expression has previously been found to be prognostic in advanced NSCLC [Citation20,Citation21] which adds to its importance in this setting. In the present study, there had been discussion with the participating study centers regarding the possibility to extract new tumor samples from the included patients, however, only in one of the centers this was deemed feasible and as that would only cover a small proportion of the included patients it was considered not enough to conduct meaningful analyses.
Another important remark on the design of the present study is whether DCT is the most appropriate cytotoxic regimen to compare the study drug AXL with. In a phase III non-inferiority designed clinical trial comparing pemetrexed 500 mg/m2 every three weeks with DCT 75 mg/m2 every three weeks in patients with NSCLC previously treated with chemotherapy, no statistically significant difference in PFS and OS was observed but the incidence of grade 3/4 neutropenia and febrile neutropenia was significantly higher in the DCT arm [Citation17]. These results have led to an increasing use of pemetrexed in combination with a platinum agent in the first-line setting or as single-agent maintenance therapy in patients with non-squamous histology [Citation22], which has reduced the availability of pemetrexed in the second-line setting. Furthermore, the present study was conducted in 19 study centers in Eastern Europe in which the experience with pemetrexed was greatly inferior to that with DCT, which also contributed to choosing DCT as the chemotherapy to compare AXL with in both AC and SCC patients.
The most commonly reported AEs in both treatment groups were neutropenia, anemia, leukopenia and asthenia. The overall incidence of AEs and especially the incidence of neutropenia were higher in the DCT group as compared with the AXL group. A total of 17 deaths related to SAEs occurred within the primary study period: 12 in the AXL group and five in the DCT group. Of nine early fatal cases in the AXL group, four cases were connected to neutropenia and/or leucopenia. These cases were reviewed in detail and it was found that three patients were treated with corticosteroids (dexamethasone) during the neutropenic periods and two of the patients were not treated with antibiotics as no fever was detected, and additionally they remained outpatients during treatment. This handling was considered inappropriate since steroids may stimulate the growth of microorganisms by causing immunosuppression, may mask fever and symptoms of infection, and do not stimulate host defense against microorganisms. The discordance between the lower incidence of grade 3 or 4 neutropenia versus the higher incidence of fatal neutropenic events in the AXL group was thought to be partly due to the daily dosing of AXL in contrast to once-every-three-weeks dosing of DCT but also due to the suboptimal management of these events, which could have been avoided with prompt antibiotic treatment in an inpatient setting.
In the AXL group, two fatal events of pulmonary hemorrhage were reported as starting on 14 and 28 days after first dose of AXL. Both patients had central squamous NSCLC tumors with tumor involvement of major blood vessels on CT which has been reported as risk factors for pulmonary hemorrhage [Citation23]. It was not possible to exclude that possible tumor shrinkage could have contributed to the bleeding.
The results of the present study illuminate the difficulty of treating patients continuously with a bone marrow toxic medication. If the neutropenia is not detected early enough with frequent blood sampling, the causative medication not stopped early enough and appropriate treatment not given early enough (antibiotics), then a manageable side effect may turn into a fatal side effect. As no neutropenia as occurred earlier that following 14 days of treatment with 400 mg BID of AXL, future studies will explore treatment duration of AXL with seven days to avoid neutropenias while on treatment. These studies will also explore seven-day dosing with doses higher than 400 mg BID of AXL.
In conclusion, the results from the present study failed to show superiority of any of the treatment groups. This lack of superiority does not preclude that the two treatments have similar efficacy considering the study was not prospectively designed to show similarity. However, both the OS and the PFS Kaplan-Meier curves have a reasonable similar profile. The lack of data on tumor IGF-1R expression unfortunately prevented further analyses of whether the anti-tumoral effect of AXL can be predicted based on the expression of the target molecule, IGF-1R. The safety profile displayed a lower incidence of leukopenia and neutropenia in the AXL group but a higher number of treatment-related fatal events. Given these results, we believe that further clinical testing of AXL, with evaluation of IGF-1R expression in tumor tissue as a predictive marker of treatment response, is warranted. Further studies of AXL in combination with treatments targeting other oncological pathways such as bevacizumab (anti-angiogenesis) or nintendanib (tyrosine-kinase inhibition) could also be an interesting approach.
Disclosure statement
Markus Jerling reports personal fees from Markus Jerling Consulting AB, during the conduct of the study; personal fees from Markus Jerling Consulting AB, outside the submitted work.
Györgi Andor reports grants from Axelar AB, during the conduct of the study.
Marcus Thuresson reports personal fees from Axelar AB, during the conduct of the study; personal fees from Axelar AB, outside the submitted work.
Elena Grechanaya reports grants from Axelar AB, during the conduct of the study.
Johan Harmenberg reports personal fees from Axelar AB, during the conduct of the study.
Alexey Maximovich reports grants from Axelar AB, during the conduct of the study.
References
- Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108.
- Noble J, Ellis PM, Mackay JA, et al. Second-line or subsequent systemic therapy for recurrent or progressive non-small cell lung cancer: a systematic review and practice guideline. J Thorac Oncol. 2006;1:1042–1058.
- Bonomi PD. Therapeutic advances in second-line treatment of advanced non-small-cell lung cancer. Clin Lung Cancer. 2004;6:154–161.
- Scagliotti GV, Novello S. The role of the insulin-like growth factor signaling pathway in non-small cell lung cancer and other solid tumors. Cancer Treat Rev. 2012;38:292–302.
- Zhang Q, Pan J, Lubet RA, et al. Targeting the insulin-like growth factor-1 receptor by picropodophyllin for lung cancer chemoprevention. Mol Carcinogen. 2015;54(Suppl 1):E129–37.
- Tabernero J, Chawla SP, Kindler H, et al. Anticancer activity of the type I insulin-like growth factor receptor antagonist, ganitumab, in combination with the death receptor 5 agonist, conatumumab. Target Oncol. 2015;10:65–76.
- Tran TN, Selinger CI, Yu B, et al. Alterations of insulin-like growth factor-1 receptor gene copy number and protein expression are common in non-small cell lung cancer. J Clin Pathol. 2014;67:985–91.
- Rosenzweig SA, Atreya HS. Defining the pathway to insulin-like growth factor system targeting in cancer. Biochem Pharmacol. 2010;80:1115–1124.
- Camidge DR, Dziadziuszko R, Hirsch FR. The rationale and development of therapeutic insulin-like growth factor axis inhibition for lung and other cancers. Clin Lung Cancer. 2009;10:262–272.
- Girnita A, Girnita L, Del Prete F, et al. Cyclolignans as inhibitors of the insulin-like growth factor-1 receptor and malignant cell growth. Cancer Res. 2004;64:236–242.
- Ekman S, Frodin JE, Harmenberg J, et al. Clinical Phase I study with an Insulin-like Growth Factor-1 receptor inhibitor: experiences in patients with squamous non-small cell lung carcinoma. Acta Oncol. 2011;50:441–447.
- Gascoigne KE, Taylor SS. How do anti-mitotic drugs kill cancer cells?. J Cell Sci. 2009;122:2579–2585.
- King H, Aleksic T, Haluska P, et al. Can we unlock the potential of IGF-1R inhibition in cancer therapy? Cancer Treat Rev. 2014;40:1096–1105.
- Fidler MJ, Shersher DD, Borgia JA, et al. Targeting the insulin-like growth factor receptor pathway in lung cancer: problems and pitfalls. Therapeut Adv Med Oncol. 2012;4:51–60.
- Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–2103.
- Fossella FV, Devore R, Kerr RN, The TAX 320 Non-Small Cell Lung Cancer Study Group, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. J Clin Oncol. 2000;18:2354–2362.
- Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–1597.
- Garassino MC, Martelli O, Broggini M, et al. Erlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): a randomised controlled trial. Lancet Oncol. 2013;14:981–988.
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. New Eng J Med. 2015;373:123–135.
- Kim JS, Kim ES, Liu D, et al. Prognostic implications of tumoral expression of insulin like growth factors 1 and 2 in patients with non-small-cell lung cancer. Clin Lung Cancer. 2014;15:213–221.
- Agullo-Ortuno MT, Diaz-Garcia CV, Agudo-Lopez A, et al. Relevance of insulin-like growth factor 1 receptor gene expression as a prognostic factor in non-small-cell lung cancer. J Cancer Res Clin Oncol. 2015;141:43–53.
- Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009;374:1432–1440.
- Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22:2184–2191.