Abstract
Background: Neoadjuvant therapy response correlates with survival in multiple gastrointestinal malignancies. To potentially augment neoadjuvant response for pancreas adenocarcinoma, we intensified treatment with stereotactic body radiotherapy (SBRT) following multi-agent chemotherapy. Using this regimen, we analyzed whether the College of American Pathology (CAP) tumor regression grade (TRG) at pancreatectomy correlated with established response biomarkers and survival.
Materials and methods: We identified borderline resectable (BRPC) and locally advanced (LAPC) pancreatic cancer patients treated according to our institutional clinical pathway who underwent surgical resection with reported TRG (n = 81, median follow-up after surgery 24.2 months). Patients had baseline CA19-9, computed tomography (CT), endoscopic ultrasound, and FDG positron emission tomography (PET)/CT then underwent multi-agent chemotherapy (79% with three cycles of gemcitabine, docetaxel and capecitabine) followed by 5-fraction SBRT. They then underwent restaging CT, PET/CT and CA19-9. Overall (OS) and progression-free (PFS) survival were estimated and compared by Kaplan–Meier and log-rank methods. Univariate ordinal logistic regression correlated TRG with baseline, restaging and change in CA19-9 and the PET maximum standardized uptake value (SUVmax).
Results: Restaging level and decrease in CA19-9 correlated with improved TRG (p = .02 for both) as did restaging SUVmax (p < .01), yet there was no TRG correlation with decrease in SUVmax (p = .10) or CT response (p = .30). The TRG groups had similar OS and PFS except the TRG 0 (complete response) group. Compared to partial response levels (TRG 1-3, median OS 33.9 months, median PFS 13.0 months), the six (7%) patients with TRG 0 had no deaths (p = .05) and only one progression (p = .03). A group of 10 (12%) TRG 1 patients with only residual isolated tumor cells had similar outcomes to the other TRG 1-3 patients.
Conclusion: Pre-operative PET-CT and CA19-9 response correlate with histopathologic tumor regression. Patients with complete pathologic response have superior outcomes, suggesting a rationale for intensification and personalization of neoadjuvant therapy in BRPC and LAPC.
Neoadjuvant chemotherapy and radiation therapy (nCRT) have improved outcomes in several gastrointestinal malignancies. For T3-4 or node positive rectal cancer, the CAO/ARO/AIO-94 (German) trial showed that nCRT yields a pathologic complete response (pCR) rate of 8%, increases the rate of sphincter preservation and decreases the rate of local recurrence (LR) compared to adjuvant chemoradiotherapy (aCRT) [Citation1]. For T2-4 or node positive resectable esophageal carcinoma, the CROSS trial nCRT regimen with pCR rate of 29% enhanced negative margin (R0) rate and overall survival (OS) compared to surgery alone [Citation2]. nCRT for T2-3 or node positive gastric cancer has also been studied by the RTOG 9904 trial in single-arm fashion, reporting a 26% pCR rate and a 77% R0 resection rate [Citation3].
This pathologic response can provide prognostic information. In the German rectal trial, the 10-year distant metastatic rate was 10.5% for pCR, 29.3% for intermediate responders and 39.6% for poor responders [Citation4]. In the RTOG 9904 gastric trial, one-year OS was 82% for pCR and 69% for those with less than a pCR [Citation3]. In an esophageal nCRT trial with 393 consecutive patients, 71 month median survival was observed for pCR versus 30 months for partial responders versus 17 months for minimal or no response [Citation5].
The role of nCRT is less defined in pancreatic cancer. The proposed benefits are aligned with those of other gastrointestinal sites such as improving R0 resection, allowing for an in vivo assessment of CRT sensitivity, and better tolerance of therapy delivered preoperatively. For upfront resectable and borderline resectable (BRPC) pancreatic cancer patients, numerous small trials have delivered neoadjuvant therapy with the additional rationale of selecting patients most likely to benefit from pancreaticoduodenectomy by observing lack of clinical progression during nCRT [Citation6]. Although pCR to neoadjuvant therapy has been uncommon in pancreatic cancer patients, limited data points to a survival benefit. One large retrospective series of neoadjuvantly treated, resected pancreatic cancers reported 11 (2.5%) pCRs of 442 patients treated with conventional radiation therapy (30 Gy in 10 fractions or 50.4 Gy in 28 fractions) and mostly single-agent chemotherapy [Citation7]. Patients with a pCR in that series enjoyed exceptional outcomes without a single failure of the initial pancreatic cancer at a median follow-up of 31 months.
It is possible that more effective neoadjuvant therapies for pancreatic cancer might raise pCR rates, leading to the long-term benefits of neoadjuvant therapy seen in esophageal and rectal cancer. Chemotherapy intensification is supported by a 2010 meta-analysis of neoadjuvant therapy for pancreatic cancer that identified improved pCR rates for combination chemotherapy (5.3%) compared to single-agent chemotherapy (2.2%) [Citation8]. Data from the metastatic setting has shown a median survival of 6.8 months with single-agent gemcitabine versus 11.4 months with FOLFIRINOX [Citation9], and 14.7 months with GTX [Citation10]. Computed tomography (CT)-based partial or complete response based on Evaluation Criteria in Solid Tumors (RECIST) reported in these studies range from 29% to 32%. In our own locally advanced pancreatic cancer (LAPC) experience, we observed more clinical response leading to resection for the folinic acid, 5-FU, irinotecan and oxaliplatin (FOLFIRINOX) regimen compared to other chemotherapy regimens [Citation11].
In addition to the integration of multi-agent chemotherapy, we have also intensified our radiation therapy regimen by employing 5-fraction stereotactic body radiation therapy (SBRT) to deliver 30 Gy over one week to the tumor with higher doses to the tumor-vessel interfaces that are at risk for margin positive resection. Compared to patients who underwent upfront resection, patients who received this neoadjuvant multi-agent chemotherapy and SBRT regimen prior to resection had similar post-operative morbidity and mortality, reduced margin positivity rate and a trend towards improved survival [Citation12]. Multi-institutional prospective data has also emerged demonstrating the safety and feasibility of a 5-fraction SBRT approach [Citation13]. Given the expanding cohort of patients treated with this regimen at our institution, we sought to identify factors that might predict or correlate with pathologic response such as patient outcomes, laboratory values and imaging metrics.
Materials and methods
Patients and initial staging
Our institutional review board approved database tracks 150 BRPC and 72 LAPC patients staged according to the National Comprehensive Cancer Network (NCCN) and treated within our institutional pathway. Patients may be entered into the pathway if are judged medically fit for resection before starting therapy and felt by the surgeon to be either borderline resectable or locally advanced but possibly resectable after imaging evidence of tumor regression. Of the 222 patients who received chemotherapy and SBRT between February 2010 and September 2015, 138 did not undergo curative-intent resection for the following reasons: 64 with LAPC were not downstaged by neoadjuvant therapy, 21 with BRPC had imaging evidence of local or distant progression, 29 with BRPC had progression identified at surgery, 15 with BRPC became medically inoperable during neoadjuvant therapy and nine were pending surgery at the time of analysis.
In total, 76 BRPC and eight LAPCs were excised, though standardized pathology grading regression was unavailable for three patients who underwent surgery elsewhere. The analyzed 81 patients had biopsy-proven American Joint Committee on Cancer (AJCC) 7th Edition clinical stage IIA-III adenocarcinoma (cT3-4, cN0-1). Patient baseline characteristics are given in .
Table 1. Patient characteristics, neoadjuvant therapies and pathologic response.
For initial staging at our institution, patients routinely undergo physical examination, blood chemistries including CA19-9, multi-detector thin-slice multi-phase contrast enhanced abdominal CT scan, positron emission tomography (PET)/CT scan, endoscopic ultrasound (EUS), specialist pathology review and presentation at multidisciplinary tumor board. For the PET/CT initial staging performed at our institution, all patients were asked to fast a minimum of six hours prior to a scan that encompassed the mid-skull to the mid-thigh region, with measurement of blood glucose prior to injection of 18F-labeled fluorodeoxyglucose (FDG). Those patients with a fasting glucose <200 mg/dl proceeded to FDG injection at least 90 minutes prior to image acquisition. The standardized uptake value (SUV)max parameter was reported by dividing the maximum activity in a voxel by the administered activity/patient mass.
As these patients often present with biliary obstruction which is relieved prior to neoadjuvant therapy by stenting if necessary, the repeat CA19-9 after relief of obstruction is used as the initial value. Thirteen patients were excluded from the CA19-9 analysis for whom pre- or post-neoadjuvant therapy CA19-9 was not available (n = 1) or pre-neoadjuvant therapy CA19-9 was not detectable (n = 12). Eleven patients were excluded from the SUVmax analysis for whom pre- or post-neoadjuvant therapy SUVmax was not available (n = 4) or pre-neoadjuvant therapy tumor SUVmax was not detectable above background (n = 7). SUVmax was calculated from the tumor by a nuclear medicine trained radiologist. In the case that there was no tumor hypermetabolism detectable (SUVmax <2.0), SUVmax was recorded as 0.
Neoadjuvant treatment and restaging
The chemotherapy and SBRT treatment schemas we have integrated into our clinical pathway have been described in detail [Citation11,Citation12]. An example case presentation is included in the online supplementary material. The most common BRPC chemotherapy regimen delivered was induction chemotherapy with three 21-day cycles of gemcitabine, docetaxel and capecitabine (GTX). All of the LAPC patients in this study received FOLFIRINOX.
Prior to radiation simulation, under EUS guidance 2–4 fiducial markers were placed into the tumor. A fluoroscopic study was then performed to gauge the amplitude of tumor motion by tracking the markers during respiration with an applied abdominal compression device. For tumor motion ≥1 cm, radiation treatment utilized respiratory gating by the Varian Real-Time Position Management system (Varian Medical Systems Inc., Palo Alto, CA). Patients were immobilized by a BodyFix cradle (Elekta, Stockholm, Sweden). Isocenter was then determined on non-contrast CT simulation scan followed by repeat four-dimensional (4D) CT with IV and oral contrast.
SBRT began at least seven days after last chemotherapy administration (median: 18 days). Five consecutive daily fractions were delivered by Varian Truebeam or Trilogy linear accelerator to gross tumor volume (GTV) within the pancreas plus motion with a 3–5mm planning tumor volume expansion. Intensity modulated radiation therapy (IMRT) was utilized to plan 25–30 Gy (minimum: 25 Gy, median: 30 Gy) to the planning tumor volume with simultaneous dose painting to up to 50 Gy (median: 40 Gy) to areas of vessel [e.g. superior mesenteric artery (SMA) or vein (SMV)] involvement by tumor. Dose was limited by organs at risk as follows: for each of duodenum, small bowel and stomach mean <25 Gy, volume receiving 30 Gy (V30) < 2 cm3, V35 Gy <0.5 cm3; each kidney mean <10 Gy, spinal cord maximum 20 Gy.
After completion of neoadjuvant treatment, BRPC and LAPC patients underwent restaging by repeat examination, CA19-9, pancreas protocol CT and PET/CT. Resectability was again determined at our multidisciplinary tumor board. The target time to surgery was 1–2 months after SBRT (median 49 days, interquartile range 14 days).
Surgery and pathology
Patients with pancreatic head tumors underwent pancreaticoduodenectomy whereas those with pancreatic body or tail tumors underwent distal pancreatectomy and splenectomy. A small percentage of patients required total pancreatectomy due to concerns of diffuse tumor infiltration or numerous intraductal mucinous papillary neoplasms (IPMNs). A vascular surgeon was available in case of need for vessel repair or resection.
Pathologic specimens were processed as follows for purposes of TRG determination. Residual gross tumor was identified and blocked for microscopic review. If a fibrous tumor bed was identified or areas of ill-defined fibrosis was identified, those areas were blocked and submitted for microscopic review. If no area suspected of being the tumor bed was identified grossly, the entire specimen was blocked for microscopic review.
Thirty-five patients required one of: repair of the SMV alone (n = 8), repair of the portal vein alone (PV, n = 7), resection and reconstruction of a portion of the SMV and/or PV (n = 17) or repair of the SMA (n = 3, 2 of whom also required repair of the SMV). Except for two PV repairs performed due to accidental vessel damage, all vessel repairs or resections were performed for suspected disease involvement close to or within the vessel that is typically indistinguishable from fibrosis. Microscopically, no tumor was identified in or close (<3 mm) to the vessel in 15 patients. Otherwise, tumor involved or was close to the vessel with >2 mm resection margins (n = 12), close margins (n = 6) or positive margins (n = 2).
The protocol from the College of American Pathology (CAP) was typically used for pathologic examination such that tumor regression grade (TRG) was measured for response to neoadjuvant therapy [Citation14]. Grading was performed by pathologists with expertise in gastrointestinal pathology. Negative margins, defined as no tumor cells at the edge of the specimen, were identified in 79 of 81 cases.
Follow-up and analysis
All charts were reviewed to identify progression, mortality and toxicity. Rates of significant side effects for radiation therapy were low, and all side effects for our overall cohort were recently reported [Citation11]. Statistical testing was performed using IBM SPSS (Windows Version 23.0; IBM Corp, Armonk, NY). Univariate ordinal logistic regressions correlated TRG with patient characteristics, neoadjuvant therapies and response metrics. Dichotomous comparisons were performed by two-sided exact Mann–Whitney U-test for continuous variables or Fisher’s exact test. Survival was estimated by the Kaplan–Meier method and compared by the log-rank (Mantel–Cox) test, with time starting at resection.
Results
For the entire cohort of 81 patients, the median follow-up from time of surgery was 24.2 months (range 0.7–54.0 months) with progression-free survival (PFS) of 17.6 months and OS of 37.5 months. Seven patients were lost to follow-up and six of those have died of uncertain causes. Two patients died of post-surgical complications. Sites of first recurrence were: 23 patients with distant metastases, two patients with local progression, two patients with regional lymph nodes, three patients with local progression and simultaneous distant metastases, and four patients with regional lymph nodes and simultaneous distant metastases.
In , we identified that survival for pCR (TRG 0) patients was exceptional compared to pathologic partial response (pPR, TRG 1-3). For the TRG 0 group (n = 6), at a median follow-up of 32.5 months in that cohort, only one patient has experienced progression. That patient, treated neoadjuvantly with neoadjuvant GTX and SBRT, developed a biopsy-proven solitary liver metastasis on imaging seven months after surgery, was treated with radiofrequency ablation and FOLFIRINOX chemotherapy, and has not developed further metastases at 27 months.
Figure 1. After multi-agent chemotherapy, SBRT and resection of pancreatic adenocarcinoma, progression-free (PFS) and overall survival (OS) are stratified by tumor regression grade (TRG) ((a) and (b), respectively) or pathologic complete response (pCR) versus partial response (pPR) ((c) and (d), respectively). PFS was better for pCR (TRG 0, median PFS not reached) compared to pPR (TRG 1-3, median PFS 13.0 months, p = .033). OS was also better for pCR (no deaths) compared to pPR (median OS 33.9 months, p = .050).
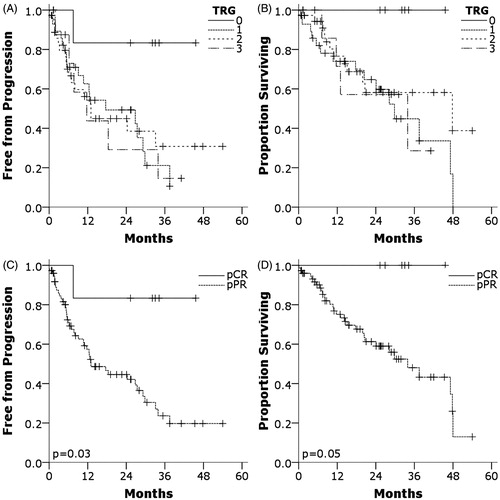
For the pPR group, median PFS and OS were 13.0 months and 33.9 months, respectively. PFS and OS were significantly better for pCR than pPR (PFS p = .033, OS p = .050). Ten patients within the TRG 1 group were identified with minimal residual disease as defined as isolated tumor cells within the specimen (individual cluster sizes less than 0.1 mm), however, their median PFS (12.6 months) and OS (29.8 months) were similar to the remainder of the pPR group.
As the pCR group had such exceptional outcomes, factors prior to surgery were analyzed to identify correlations to pCR. In a number of factors are listed that were correlated against pCR/pPR and TRG: CA19-9, SUVmax and RECIST.
Table 2. CA19-9, SUVmax and RECIST criteria for the 81 analyzed specimens.
A significant correlation was found between pCR and pPR for post-neoadjuvant therapy CA19-9 level, but not for change in CA19-9, post-neoadjuvant therapy SUVmax, change in SUVmax, or CT response by RECIST 1.0 criteria (). All patients found to have a pCR had a post-therapy CA19-9 value less than half (18.5 U/ml) of normal. Significantly fewer in the pPR group, 23 (36%) of 63, had a CA19-9 value of less than half normal (p = .008). TRG was then correlated with CA19-9 or SUVmax to identify a trend across TRG levels. Post-neoadjuvant therapy CA19-9 (log10 transformed), percent reduction in CA19-9 and post-neoadjuvant therapy SUVmax significantly correlated with TRG. These are shown in .
Figure 2. Individual data points (circles), medians (diamonds), and 95% confidence intervals (bars) for post-neoadjuvant therapy SUVmax (A), SUVmax percent reduction before and after neoadjuvant therapy (B), post-neoadjuvant therapy CA19-9 (C), and CA19-9 percent reduction before and after neoadjuvant therapy. Significance levels by ordinal logistic regression are given for each plot (p < .05 for A, C, and D, p = .095 for B).
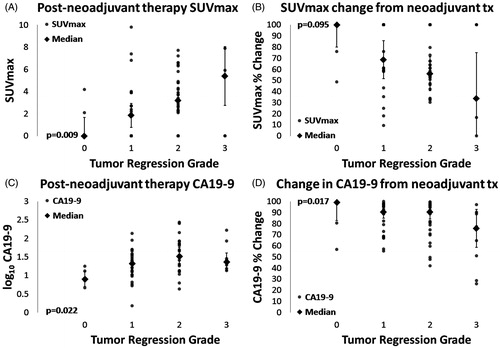
Table 3. Correlations of TRG to CA19-9, SUVmax and RECIST response.
The 12 patients with undetectable CA19-9 at presentation had similar OS (median 37.5 months, p = .98) and PFS (median 12.6 months, p = .77) compared to the overall group. They had similar levels of response by TRG (1 of 12 with pCR).
Discussion
The goal of the neoadjuvant multi-agent chemotherapy and SBRT approach to BRPC and LAPC has been an improvement in tumor response and patient outcomes. In this series we identified a 7.4% pCR rate, higher than most studies of conventional single-agent chemotherapy and non-SBRT radiotherapy [Citation8]. Patients achieving pCR had markedly improved outcomes (no deaths at 32.5 months) compared to patients who did not achieve a pCR (median survival 33.9 months). We have previously reported the feasibility and safety of combining both sequential multi-agent chemotherapy (mostly GTX or FOLFIRINOX) and SBRT in a neoadjuvant and/or definitive approach for 159 patients with BRPC and LAPC [Citation11].
Chaterjee et al. found six (2.7%) pCRs within 223 resected, neoadjuvantly treated pancreatic cancers [Citation15]. Although the Kaplan–Meier figures for CAP TRG in their manuscript appear quite similar to this work, they observed somewhat better survival for TRG 1 than TRG 2 and 3. In contrast, we were unable to identify a minimal residual tumor group with improved prognosis in our study. We also compared TRG 0-1 versus TRG 2-3 or TRG 0 and isolated tumor cells versus remaining TRG 1 patients and TRG 2-3 patients, but these were not significantly different from one another (data not shown). This may reflect variability in the measurement of pathologic features such as TRG that may require future consideration for interpretation across studies [Citation16]. One such consideration is that in our experience there is interobserver variability using the CAP system. Although a TRG of 0 is reproducible for a given specimen, TRGs of 1, 2 or 3 are more difficult to replicate. A TRG of 1 is defined as minimal residual cancer using the CAP, but what this means to an individual pathologist may vary. This may explain why the TRG 1 patients did not perform better than the TRG 2 or 3 patients in our study. Additionally, it is possible that this reflects differences in the types of neoadjuvant therapies used or that our smaller series does not have the power to detect an improved prognosis group in TRG 1.
Our analysis found that post-treatment PET correlated with TRG, but not PET response. This likely reflects a limitation of PET in this study. Pre-therapy PET scans and CA19-9 values can be falsely elevated by inflammation before the relief of biliary obstruction. In this study, CA19-9 was easily and relatively cheaply repeated after the relief of biliary obstruction, and post-obstruction values were used for analysis. However, the PET was often not repeated until after completion of neoadjuvant therapy due to its cost and current clinical use in our institution to rule out distant metastases. Another limitation of PET reflected in this series is that 5–10% of pancreatic carcinomas are not detectable by FDG PET [Citation17]. Further, SUVmax can have significant measurement variance in clinical practice [Citation18]. Alternatively, FDG PET tumor metabolism can also be measured by metabolic tumor volume (MTV) and total lesion glycolysis (TLG). Dholakia et al. correlated high MTV and/or TLG before SBRT with inferior OS for LAPC patients [Citation19].
Nevertheless, with appropriate quality control PET response to guide neoadjuvant therapy or surgery could be considered. One interesting study of LAPC included 18 patients who underwent two cycles of induction chemotherapy with cisplatin, cytarabine and 5-FU, with PET before and after the first cycle of chemotherapy, followed by CRT [Citation20]. The two patients in that study who had a >50% PET response after one cycle of chemotherapy went on to complete surgical resection compared to only one of 16 in <50% response group.
We found correlations in this dataset between TRG and CA19-9 level after neoadjuvant therapy or change in CA19-9 level due to neoadjuvant therapy. More strikingly, Boone et al. reported pCR in five (6%) of 78 patients with resectable, BRPC or LAPC, and these patients all had a >90% decrease in CA19-9 [Citation21]. In the current report, decrease in CA19-9 did not correlate as strongly with response. This may be because we only excluded patients with undetectable levels of CA19-9, noting that patients with mildly elevated or CA19-9 levels within the high range of normal also responded to therapy with a decrease in CA19-9. Performing the analysis only with patients with elevated CA19-9 did not significantly alter the results. Conversely, Katz et al. found no correlation between treatment effect score and 59 resectable pancreatic cancer patients with pre- and post-therapy CA19-9 levels, although a different tumor regression scoring system was used [Citation22].
A limitation of CA19-9 in pancreatic cancer is that up to one third of patients are Lewis-antigen negative and express no or limited quantities of CA19-9 [Citation17]. Future work aims to determine additional tumor biomarkers that correlate with tumor response or outcomes with the goal of better tailoring neoadjuvant therapy and definitive surgery for pancreatic cancer. Although we have been unable to identify specific cut points that indicate resectability or complete response, it is possible that combinations of laboratory tests, biopsy markers or other imaging techniques might permit selection of patients for resection or selection of chemotherapy regimens or radiation techniques.
In conclusion, pCR of BPRC or LAPC to intensified neoadjuvant therapy remains uncommon, but portends an excellent prognosis. CA19-9 levels before surgery correlate most strongly with pCR response. Correlations are also observed between TRG and SUVmax after neoadjuvant therapy as well as reduction in CA19-9 due to neoadjuvant therapy. Future work should attempt to increase therapy response, identify additional markers of response and/or apply markers for adaptation of therapy.
IONC_1256497_supplementary.docx
Download MS Word (1.6 MB)Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740.
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084.
- Ajani JA, Winter K, Okawara GS, et al. Phase II trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma (RTOG 9904): quality of combined modality therapy and pathologic response. J Clin Oncol. 2006;24:3953–3958.
- Fokas E, Liersch T, Fietkau R, et al. Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: updated results of the CAO/ARO/AIO-94 trial. J Clin Oncol. 2014;32:1554–1562.
- Donohoe CL, O'Farrell NJ, Grant T, et al. Classification of pathologic response to neoadjuvant therapy in esophageal and junctional cancer: assessment of existing measures and proposal of a novel 3-point standard. Ann Surg. 2013;258:784–792.
- Hoffe S, Rao N, Shridhar R. Neoadjuvant vs adjuvant therapy for resectable pancreatic cancer: the evolving role of radiation. Semin Radiat Oncol. 2014;24:113–125.
- Zhao Q, Rashid A, Gong Y, et al. Pathologic complete response to neoadjuvant therapy in patients with pancreatic ductal adenocarcinoma is associated with a better prognosis. Ann Diagn Pathol. 2012;16:29–37.
- Gillen S, Schuster T, Meyer Zum Buschenfelde C, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7:e1000267.
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825.
- Fine RL, Fogelman DR, Schreibman SM, et al. The gemcitabine, docetaxel, and capecitabine (GTX) regimen for metastatic pancreatic cancer: a retrospective analysis. Cancer Chemother Pharmacol. 2008;61:167–175.
- Mellon EA, Hoffe SE, Springett GM, et al. Long-term outcomes of induction chemotherapy and neoadjuvant stereotactic body radiotherapy for borderline resectable and locally advanced pancreatic adenocarcinoma. Acta Oncol. 2015;54:979–985.
- Mellon EA, Strom TJ, Hoffe SE, et al. Favorable perioperative outcomes after resection of borderline resectable pancreatic cancer treated with neoadjuvant stereotactic radiation and chemotherapy compared with upfront pancreatectomy for resectable cancer. J Gastrointest Oncol 2016;7:547–555.
- Herman JM, Chang DT, Goodman KA, et al. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer. 2015;121:1128–1137.
- Callery MP, Chang KJ, Fishman EK, et al. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1727–1733.
- Chatterjee D, Katz MH, Rashid A, et al. Histologic grading of the extent of residual carcinoma following neoadjuvant chemoradiation in pancreatic ductal adenocarcinoma: a predictor for patient outcome. Cancer. 2012;118:3182–3190.
- Verbeke C, Lohr M, Karlsson JS, et al. Pathology reporting of pancreatic cancer following neoadjuvant therapy: challenges and uncertainties. Cancer Treat Rev. 2015;41:17–26.
- Pakzad F, Groves AM, Ell PJ. The role of positron emission tomography in the management of pancreatic cancer. Semin Nucl Med. 2006;36:248–256.
- Kumar V, Nath K, Berman CG, et al. Variance of SUVs for FDG-PET/CT is greater in clinical practice than under ideal study settings. Clin Nucl Med. 2013;38:175–182.
- Dholakia AS, Chaudhry M, Leal JP, et al. Baseline metabolic tumor volume and total lesion glycolysis are associated with survival outcomes in patients with locally advanced pancreatic cancer receiving stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2014;89:539–546.
- Choi M, Heilbrun LK, Venkatramanamoorthy R, et al. Using 18F-fluorodeoxyglucose positron emission tomography to monitor clinical outcomes in patients treated with neoadjuvant chemo-radiotherapy for locally advanced pancreatic cancer. Am J Clin Oncol. 2010;33:257–261.
- Boone BA, Steve J, Zenati MS, et al. Serum CA 19-9 response to neoadjuvant therapy is associated with outcome in pancreatic adenocarcinoma. Ann Surg Oncol. 2014;21:4351–4358.
- Katz MH, Varadhachary GR, Fleming JB, et al. Serum CA 19-9 as a marker of resectability and survival in patients with potentially resectable pancreatic cancer treated with neoadjuvant chemoradiation. Ann Surg Oncol. 2010;17:1794–1801.
