Abstract
Background: Meningiomas are usually slow growing, well circumscribed intracranial tumors. In symptom-free cases observation with close follow-up imaging could be performed. Symptomatic meningiomas could be surgically removed and/or treated with radiotherapy. The study aimed to evaluate the volumetric response of intracranial meningiomas at different time points after photon, proton, and a mixed photon and carbon ion boost irradiation.
Patients and methods: In Group A 38 patients received proton therapy (median dose: 56 GyE in 1.8–2 GyE daily fractions) or a mixed photon/carbon ion therapy (50 Gy in 2 Gy daily fractions with intensity modulated radiotherapy (IMRT) and 18 GyE in 3 GyE daily dose carbon ion boost). Thirty-nine patients (Group B) were treated by photon therapy with IMRT or fractionated stereotactic radiotherapy technique (median dose: 56 Gy in 1.8–2 Gy daily fractions). The delineation of the tumor volume was based on the initial, one- and two-year follow-up magnetic resonance imaging and these volumes were compared to evaluate the volumetric tumor response.
Results: Significant tumor volume shrinkage was detected at one- and at two-year follow-up both after irradiation by particles and by photons. No significant difference in tumor volume change was observed between photon, proton or combined photon plus carbon ion boost treated patients. WHO grade and gender appear to be determining factors for tumor volume shrinkage.
Conclusion: Significant volumetric shrinkage of meningiomas could be observed independently of the applied radiation modality. Long-term follow-up is recommended to evaluate further dynamic of size reduction and its correlation with outcome data.
Meninigiomas are usually slow growing, well circumscribed and benign tumors deriving from arachnoidal cells. They are the most common primary non-glial brain tumors in adults and account for 15–30% of all intracranial neoplasms [Citation1,Citation2]. Despite their generally benign character, they are often neighboring or infiltrating critical neurovascular structures and their growth can cause neurological or neurocognitive deficits leading to a significant worsening in quality of life.
Gross total resection (GTR) provides long-term recurrence-free survival in many cases [Citation3,Citation4]. If only subtotal resection (STR) can be achieved or in cases of grade II tumors, adjuvant radiotherapy (RT) should be considered. In cases of anaplastic meningiomas RT should be always a part of the therapy [Citation5,Citation6]. Beside surgical resection highly conformal RT has also become a primary treatment alternative over the last decades. Due to improvements in treatment planning and technical application of high precision photon RT, fractionated stereotactic radiotherapy (FSRT) and intensity modulated radiotherapy (IMRT) have been well established. These techniques showed convincing local control (75–100%) and very low side effect rates [Citation7–10].
Since November 2009, protons and carbon ions are available at the Heidelberg Ion-Beam Therapy Center (HIT). RT with charged particles offers high precision dose deposition in a defined depth (Bragg peak). The raster scanning technique allows maximal homogeneous irradiation of the tumor while minimizing the dose to the surrounding healthy structures as organs at risk (OARs). Furthermore, the high linear energy transfer (LET) of carbon ions causes dense ionization resulting in an increased relative biological effectiveness (RBE) in radiorersistant tumors, which is about 2–5 times higher than in photon therapy [Citation11].
As a result of the mostly benign character of meningiomas and a relatively long life expectancy of patients suffering from this disease, it is essential to avoid radiation-induced late side effects. With highly conformal RT a maximum prevention of the OARs can be achieved. As meningiomas are known to be relatively radioresistant and are often adjacent to critical nervous structures, ion therapy is expected to be particularly suitable for their treatment [Citation12]. Our previous data have already demonstrated promising results after irradiation of meningiomas by particles [Citation13–15].
In the past, several groups have evaluated the volumetric response of intracranial meningiomas after photon RT [Citation16,Citation17]. Up to now, no work has been published using particle therapy. Hence, the aim of this study was to evaluate the volumetric response after both proton and carbon ion irradiation and to compare the results with photon irradiation.
Patients and methods
Two groups of 77 patients, chosen from a large database [Citation18], suffering from inoperable (even biopsy was not feasible; grade of meningioma is unknown), residual or recurrent meningioma, treated in two time periods with different radiation modalities, were analyzed and compared retrospectively. The performance of this study was approved by the local ethics committee (Ethikkommission Medizinische Fakultät Heidelberg). In the first group (Group A) patients were randomly chosen from the database [Citation18]. They received proton therapy or carbon ion boost according to a defined protocol [Citation15]. Patients with similar characteristics (age, gender, tumor volume, etc.) treated by photon therapy (IMRT or FSRT) were chosen (Group B) to compare the dynamics of tumor response.
Group A consists of 38 patients who were treated at the HIT between September 2010 and January 2012 due to inoperable (10/38), residual (6/38) or recurrent (22/38) meningiomas. Histological WHO grade was unknown in 10/38, grade I in 17/38, grade II in 10/38 and grade III in 1/38 patients. Median age at the time of RT was 52.5 years (range 32.1–76.8 years). Male to female ratio was 9:29. The tumors were located at the skull base in 31/38, attached to the olfactory tract in 4/38, at the falx in 2/38 or in the orbit in 1/38 patients. Proton RT was delivered to the macroscopic tumor with a safety margin in benign cases (unknown and grade I 27/38) with a median dose of 56 GyE (range 54–58 Gy) in 1.8 or 2 GyE daily fractions. For high grade meningiomas (grade II and III 11/38) a mixed photon/carbon ion scheme according to the MARCIE protocol was used: 50 Gy in 2 Gy daily fractions with IMRT and 18 GyE in 3 GyE daily dose carbon ion boost to the macroscopic tumor [Citation15].
In the past, several hundred patients were treated by IMRT or FSRT for their intracranial meningioma at the Department of Radiation Oncology of the University Hospital Heidelberg [Citation6,Citation9,Citation12]. From these, 39 patients were selected who had been treated between November 2000 and July 2009 and matched best regarding the clinical parameters (age, gender, tumor volume, etc.) (Group B). They were irradiated because of inoperable (12/39), residual (10/39) or recurrent (17/39) meningiomas. Histological grade was unknown in 12/39, grade I in 16/39, grade II in 7/39 and grade III in 4/39 patients. Median age at the time of RT was 55.2 years (range 20.6–80.8 years). Male to female ratio was 11:28. Meningiomas were located at the skull base in 25/39, at the convexity in 5/39, in the cavernous sinus in 4/39, at the falx in 2/39, on the optic nerve in 2/39 or at the craniocervical junction in 1/39 patients. IMRT or FSRT was applied with a median dose of 56 Gy (range 39.6–60 Gy) in 1.8 or 2 Gy daily fractions ().
Table 1. Patients' characteristics.
Patients were individually affixed with special head masks (Scotch Cast- or Thermoplast-masks) as described previously [Citation9,Citation14]. For delineation of OARs and gross tumor volume (GTV) a trimodal image fusion of a contrast-enhanced computed tomography (CT) scan with 3 mm slices, a T1 weighted, contrast-enhanced magnetic resonance imaging (MRI) and a DOTATOC-positron emission tomography (PET) was used, as this procedure was shown to be benificial in many cases [Citation13,Citation19–21].
For photon and proton RT the clinical target volume (CTV) included the GTV as well as a safety margin depending on histology was added as described previously (1–3 mm in cases of low grade pathology and 10–20 mm in atypical and anaplastic tumors) [Citation6]. Anatomical borders were respected.
According to the MARCIE trial, a 5 mm safety margin was given to the GTV for the carbon ion boost RT. For all patients the planning target volume (PTV) was calculated with an additional margin of 2–3 mm in Thermoplast- or 1–2 mm in Scotch Cast masks-setup ().
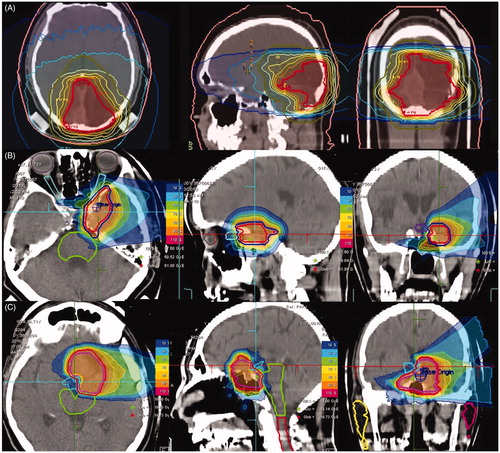
Figure 1. Treatment plans of three patients: IMRT (A), protons (B), and carbon ion boost according to MARCIE protocol (C).
After RT, patients were enrolled in a continuous and regular follow-up program: thorough clinical-neurological examination and contrast-enhanced MRI following our in-house standard. The first- and second-year follow-up MRIs were compared with the pre-therapeutic one in each patient to evaluate the volumetric tumor response for this study. Therefore, the GTV was delineated on the initial pre-therapeutical MRI as well as on the first- and second-year follow-up MRIs in cooperation of two experienced radiation oncologists using Siemens Dosimetrist (Siemens Medical Solutions, Concord, CA). Afterwards, these three GTVs (pre-therapeutical, first- and second-year follow-up) were compared and tumor volumes (TV) were calculated in cm3 (Masterplan Oncentra, Nucletron, Columbia, MD).
The statistical analysis was performed with SPSS 20. Paired and two samples t-tests as well as ANOVA tests were done.
Results
Every patient completed RT without a long interruption. Detailed treatment outcome of the patients has been published from our institution in the past [Citation6,Citation9,Citation12,Citation13,Citation15,Citation22].
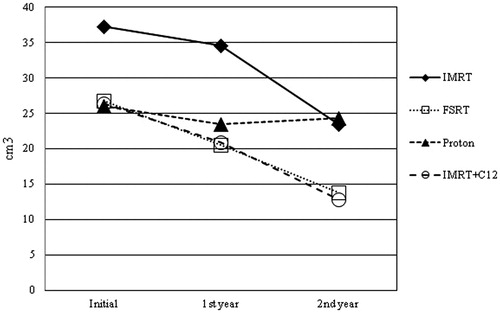
In Group A, 27 patients were treated by proton RT. The mean initial TV was Vmean = 26.1 ± 22.2 cm3. There was a significant absolute TV shrinkage after one year (Vmean = 23.5 ± 19.8 cm3; Vchange mean = −3.7 ± 4.6 cm3, p = .001). At the two-year follow-up a steady state could be observed compared to the volumes after one year (Vmean = 24.3 ± 20.7 cm3). Eleven patients were treated by carbon ion boost combined with IMRT. The mean inital TV was Vmean = 26.5 ± 15.4 cm3. There was a siginificant absolute TV shrinkage at one-year follow-up (Vmean = 20.9 ± 14.4 cm3; Vchange mean = −5.7 ± 5.6 cm3, p = .011). At two-year follow-up the contrast enhancing volume had decreased (Vmean = 12.9 ± 10.0 cm3), however, this shrinkage was not significant (p = .083). There was no significant difference in TV changes between combined IMRT plus carbon ion boost and proton-treated patients.
In Group B, 16 patients were treated by IMRT. The mean initial TV was Vmean = 37.3 ± 29.5 cm3. There was a significant absolute TV shrinkage both after one year (Vmean = 34.6 ± 28.0 cm3; Vchange mean = −4.3 ± 4.1 cm3, p = .003) and at the two-year follow-up (Vmean = 23.5 ± 17.5 cm3; Vchange mean = −9.0 ± 5.2 cm3, p = .017). There was a significant absolute shrinkage after two years compared to the one-year follow-up as well (Vchange mean = −3.4 ± 1.5 cm3, p = .020).
Twenty-three patients were treated by FSRT. The mean inital TV was Vmean = 26.7 ± 23.1 cm3. There was a siginificant absolute TV shrinkage both after one year (Vmean = 20.5 ± 14.3 cm3; Vchange mean = −7.0 ± 14.7 cm3, p = .042) and at the two-year follow-up (Vmean = 13.9 ± 10.0 cm3; Vchange mean = −4.7 ± 3.9 cm3, p = .001). There was a significant absolute shrinkage at two-year compared to one-year follow-up as well (Vchange mean = −1.3 ± 1.8 cm3, p = .038). There was no significant difference in TV changes between IMRT- and FSRT-treated patients ( and , ).
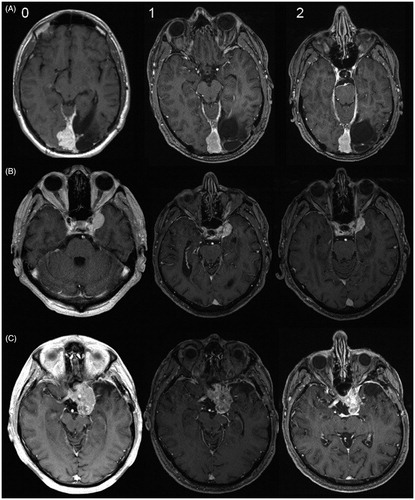
Figure 3. (0) Baseline MRI (for treatment planning) and follow-up MRIs after one (1) and two years (2) after RT by photons (A), protons (B) and after a combined RT by photons and carbon ions according to the MARCIE-protocol (C).
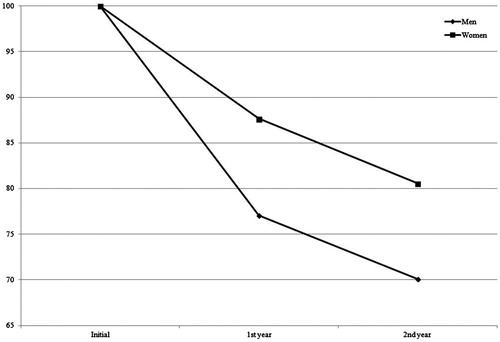
A significant absolute TV shrinkage was found in male as well as in female patients. In men (20/77) the mean inital TV was Vmean = 40.6 ± 31.4 cm3, after one year Vmean = 34.0 ± 27.4 cm3 (p = .018) and after two years Vmean = 19.5 ± 13.2 cm3 (p < .0001). In women (57/77) the mean inital TV was Vmean = 24.5 ± 18.4 cm3, at one-year follow-up Vmean = 21.6 ± 16.4 cm3 (p < .001) and two-year follow-up Vmean = 17.5 ± 16.2 cm3 (p < .0001). Men showed a significant higher shrinkage after irradiation by both modalities and at both follow-up examinations. In male patients the Vchange mean was −10.1 ± 15.8 cm3 and −7.8 ± 4.6 cm3 after one and two years, respectively. In women it was Vchange mean = −3.5 ± 4.3 cm3 and −3.9 ± 3.3 cm3 (Mann-Whitney U-test p = .028, p = .022) (). Therefore, gender was found to be an independent predictive factor for TV change.
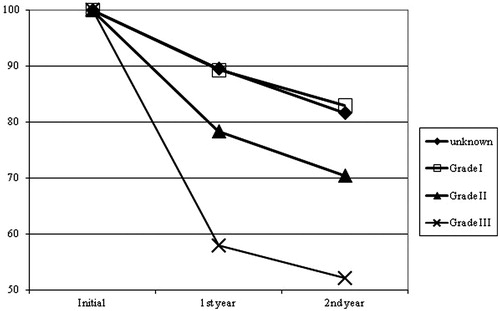
In patients with grade III meningioma (5/77, initial Vmean = 31.4 ± 21.5 cm3) we observed significantly higher relative TV shrinkage in comparison to patients with unknown histology (22/77, initial Vmean = 22.2 ± 15.1 cm3) as well as to patients with grade I meningiomas (33/77, initial Vmean = 30.0 ± 25.1 cm3), both at one-year (p = .045 and p = .038) and two-year follow-up (p = .010 and p = .012). The mean relative size of the residual, contrast enhancing TV was 58.0 ± 22.9% and 52.1 ± 13.5% in the grade III cases after one and two years. In patients with unknown histology the residual TV was 89.6 ± 19.9% and 81.7 ± 6.6% as well as 89.3 ± 17.3% and 83.0 ± 13.6% in grade I meningiomas, respectively. Patients with grade II meningioma showed a higher tumor shrinkage at one- and two-year follow-up than patients with unknown or with grade I meningioma, but less tumor volume reduction than grade III. No significancy could be performed between the group of grade II meningioma in comparison with the unknown or grade I and grade III tumors ().
Neither age, radiation modality (photon vs. particle), initial TV nor operability were found to be significant independent predictive factors for volumetric response at the two-year follow-up.
Discussion
The aim of this retrospective study was to compare the volumetric changes of meningiomas one and two years after treatment by different RT modalities. This is the first evaluation of volumetric response of intracranial meningiomas after irradiation by particles and the first analysis comparing the change of TV after different RT modalities.
We could show that both high precision photon RT and irradiation by charged particles alone or in combination with photon RT lead to a significant measurable TV reduction in first and second year after RT. In the patient group where RT was performed with protons a steady state could be observed between the volumes at one and two years contoured on the follow-up MRI images. Further follow-up might provide more detailed results about volumetric changes. In all but one patient a size reduction of the TV was detected both on the one- and two-year follow-up MRIs. Mean tumor shrinkage of about 25% was measured after two years over all treatment modalities. In patients with tumor-related neurological disorders caused by affection of sensitive neighboring structures this seems to be too slow to avoid persistent deficits. In these cases RT does not seem to be the first treatment of choice, if an early neurosurgical resection could prevent the patient from long-term sequelae [Citation1,Citation4]. In patients with inoperable tumors or after STR RT can be considered as a primary or as an adjuvant treatment [Citation1,Citation7]. In grade III tumors adjuvant RT significantly improves progression-free (PFS) and overall survival (OS) even if GTR could be performed [Citation23]. In grade II meningiomas treatment concepts are still controversial. If STR was feasible, RT can be delivered with an additive approach, or it can be delayed until progression [Citation5,Citation24].
Astner et al. [Citation16] described TV shrinkage by evaluating the initial and follow-up volumetric data of irradiated meningiomas. They measured the TVs of 53 FSRT and six SRT-treated patients. For the FSRT-treated group the mean follow-up period was 49.5 months (range 11–95 months) and the mean relative size reduction was 25.5% (range 0–69.6%). They observed significant relative tumor shrinkage of 17% after 24, 23% after 24–48, 30% after 48–72 and 26% after more than 72 months, respectively. However, they did not observe any additional reduction after more than 72 months, which might be the effect of a smaller number of available MRIs in further follow-up. A significant TV shrinkage was detected by Henzel et al. as well [Citation17]. They prospectively observed 84 FSRT-treated patients with grade I meningiomas. They found a significant linear reduction of the volumes on every follow-up after 6, 12, 18, 24, and 36 months with 16.6%, 24.5%, 27.9%, 33.2% and 36.0% respectively. Henzel et al. predict a continuous decrease towards a steady state which they still could not describe.
These results are comparable to our data. In our FSRT-treated patients we observed similar significant TV shrinkage. The relative size of the residual contrast enhancing TV was 84.0 ± 22.9% (p = .042) and 76.9 ± 14.6% (p = .001) at one- and two-year follow-up, respectively. Similar volumetric decreases were observed after irradiation with the other applied RT modalities.
This is the first study that compares the TV shrinkage of meningiomas according to different RT modalities. We could not demonstrate any significant difference in volume reduction depending on the applied radiation quality. IMRT and FSRT were administered with the similar median dose (56 Gy, range 39.6–60 Gy) in 1.8 or 2 Gy daily fractions. These photon techniques have already shown their positive effect on local control, disease-free (DFS) and OS rates without major toxicity [Citation5–10,Citation12,Citation23]. Henzel et al. could not demonstrate any correlation between the TV shrinkage and the delivered radiation doses [Citation17]. They applied a median dose of 56 Gy (range 50.4–59.4 Gy). Our delivered equivalent proton irradiation doses were similar to the photon protocols (median 56 GyE; range 54–58 Gy) applied in 1.8 or 2 GyE daily fractions. Carbon ions were applied in a mixed photon/carbon ion scheme according to the MARCIE protocol [Citation15]. Although a carbon ion boost combined with photon irradiation gave the option of dose escalation in the target volume, we could not observe higher TV reduction. Nevertheless, we have to underline the benefits of particle therapy due to dose reduction in the neighboring OARs. As patients suffering from meningiomas have long lifetime expectancy, the avoidance of late side effects such as second malignancies, neurocognitive deficits or vascular events is particularly important [Citation25].
For the first time volumetric tumor regression after RT according to the WHO grade was analyzed. The incidence of grade II tumors is approximately 5–7% of all meningiomas and the occurrence of grade III tumors is 0.17/100 000/year [Citation1]. Among our patients the incidence of grade III meningiomas was 6.5% (5/77), which is higher than expected. Interestingly, we observed significantly higher TV shrinkage in these patients both at one- and two-year follow-up in comparison to grade I meningiomas and the group of patients with the unknown histology (). The reason could be the increased proliferation and decreased regeneration of anaplastic cells leading to higher radiation sensitivity. On the contrary, higher grade is stated to be a negative prognostic factor in respect of DFS and OS [Citation1,Citation3,Citation6,Citation12].
Both men and women showed significant TV reduction at both follow-up examinations. Interestingly, we have found significantly higher TV shrinkage among men. Although male gender is an unfavorable prognostic factor in respect of OS [Citation1], in our patient cohort men presented higher TV shrinkage (), however, this might not correlate with the survival.
We did not observe any difference in TV shrinkage after RT in correlation to operability. In our patient group there were 22/77 inoperable (mean initial TV Vmean = 22.2 ± 15.1 cm3) and 55/77 previously operated patients (mean initial TV Vmean = 31.3 ± 25.6 cm3). Both groups showed TV shrinkage at the follow-up examinations: the size of the residual volumes were 89.6 ± 19.9% and 81.7 ± 6.6% for inoperable and 83.9 ± 21.6% and 75.0 ± 19.1% for operated patients at one- and two- year follow-up, respectively. These findings correspond with Astner et al. [Citation16] and Henzel et al. [Citation17] who also could not find any predictive correlation between TV shrinkage after RT and operability.
Conclusion
Evaluation of contrast enhancing tumor size after RT of meningiomas using MRI-based volumetric measurements is a precise method to detect tumor regression. We have observed significant TV shrinkage independently of the applied radiation modality. TV shrinkage can be detected one and two years after irradiation. WHO grade and gender appear to be determining factors for TV shrinkage. Neither previous surgery, age nor size of the initial TV influence TV reduction. Further long-term follow-up is recommended to determine whether size reduction correlates with DFS and OS rates.
Disclosure statement
The authors declare no conflict of interest.
References
- Marosi C, Hassler M, Roessler K, et al. Meningioma. Crit Rev Oncol Hematol. 2008;67:153–171.
- Bondy M, Ligon BL. Epidemiology and etiology of intracranial meningiomas: a review. J Neurooncol. 1996;29:197–205.
- Beks JWF, Dewindt HL. The recurrence of supratentorial meningiomas after surgery. Acta Neurochir. 1988;95:3–5.
- Demonte F, Smith HK. Outcome of aggressive removal of cavernous sinus meningiomas. J Neurosurg. 1994;81:245–251.
- Walcott BP, Nahed BV, Brastianos PK, et al. Radiation treatment for WHO grade II and III meningiomas. Front Oncol. 2013;3:227.
- Adeberg S, Hartmann C, Welzel T, et al. Long-term outcome after radiotherapy in patients with atypical and malignant meningiomas-clinical results in 85 patients treated in a single institution leading to optimized guidelines for early radiation therapy. Int J Radiat Oncol Biol Phys. 2012;83:859–864.
- Debus J, Wuendrich M, Pirzkall A, et al. High efficacy of fractionated stereotactic radiotherapy of large base-of-skull meningiomas: long-term results. J Clin Oncol. 2001;19:3547–3553.
- Milker-Zabel S, Zabel-Du Bois A, Huber P, et al. Fractionated stereotactic radiation therapy in the management of benign cavernous sinus meningiomas: long-term experience and review of the literature. Strahlenther Onkol. 2006;182:635–640.
- Combs SE, Adeberg S, Dittmar JO, et al. Skull base meningiomas: long-term results and patient self-reported outcome in 507 patients treated with fractionated stereotactic radiotherapy (FSRT) or intensity modulated radiotherapy (IMRT). Radiother Oncol. 2013;106:186–191.
- Compter I, Zaugg K, Houben RMA, et al. High symptom improvement and local tumor control using stereotactic radiotherapy when given early after diagnosis of meningioma A multicentre study. Strahlenther Onkol. 2012;188:887–893.
- Combs SE, Bohl J, Elsasser T, et al. Radiobiological evaluation and correlation with the local effect model (LEM) of carbon ion radiation therapy and temozolomide in glioblastoma cell lines. Int J Radiat Biol. 2009;85:126–137.
- Milker-Zabel S, Zabel A, Schulz-Ertner D, et al. Fractionated stereotactic radiotherapy in patients with benign or atypical intracranial meningioma: long-term experience and prognostic factors. Int J Radiat Oncol Biol Phys. 2005;61:809–816.
- Combs SE, Welzel T, Habermehl D, et al. Prospective evaluation of early treatment outcome in patients with meningiomas treated with particle therapy based on target volume definition with MRI and 68Ga-DOTATOC-PET. Acta Oncol. 2013;52:514–520.
- Combs SE, Kalbe A, Nikoghosyan A, et al. Carbon ion radiotherapy performed as re-irradiation using active beam delivery in patients with tumors of the brain, skull base and sacral region. Radiother Oncol. 2011;98:63–67.
- Combs SE, Edler L, Burkholder I, et al. Treatment of patients with atypical meningiomas Simpson grade 4 and 5 with a carbon ion boost in combination with postoperative photon radiotherapy: the MARCIE Trial. BMC Cancer. 2010;10:8.
- Astner ST, Theodorou M, Dobrei-Ciuchendea M, et al. Tumor shrinkage assessed by volumetric mri in the long-term follow-up after stereotactic radiotherapy of meningiomas. Strahlenther Onkol. 2010;186:423–429.
- Henzel M, Gross MW, Hamm K, et al. Significant tumor volume reduction of meningiomas after stereotactic radiotherapy: results of a prospective multicenter study. Neurosurgery. 2006;59:1188–1194.
- Kessel KA, Bohn C, Engelmann U, et al. Five-year experience with setup and implementation of an integrated database system for clinical documentation and research. Comput Methods Programs Biomed. 2014;114:206–217.
- Gehler B, Paulsen F, Oksuz MO, et al. Ga-68 -DOTATOC-PET/CT for meningioma IMRT treatment planning. Radiat Oncol. 2009;4:8.
- Milker-Zabel S, Zabel-Du Bois A, Henze M, et al. Improved target volume definition for fractionated stereotactic radiotherapy in patients with intracranial meningiomas by correlation of CT, MRI, and Ga-68 – DOTATOC-PET. Int J Radiat Oncol Biol Phys. 2006;65:222–227.
- Thorwarth D, Henke G, Muller AC, et al. Simultaneous (68)Ga-DOTATOC-PET/MRI for IMRT treatment planning for meningeoma: first experience. Int J Radiat Oncol Biol Phys. 2011;81:277–283.
- Combs SE, Hartmann C, Nikoghosyan A, et al. Carbon ion radiation therapy for high-risk meningiomas. Radiother Oncol. 2010;95:54–59.
- Park HJ, Kang HC, Kim IH, et al. The role of adjuvant radiotherapy in atypical meningioma. J Neurooncol. 2013;115:241–247.
- Maclean J, Fersht N, Short S. Controversies in radiotherapy for meningioma. Clin Oncol (R Coll Radiol). 2014;26:51–64.
- Combs SE, Laperriere N, Brada M. Clinical controversies: proton radiation therapy for brain and skull base tumors. Semin Radiat Oncol. 2013;23:120–126.