Abstract
Background: Traditional rehabilitative approaches to perioperative cancer care have focused on the postoperative period to facilitate the return to presurgical baseline conditions. However, there is some realization that the preoperative period can be a very effective time for intervention as the patients are more amenable to target their physiological condition to prepare to overcome the metabolic cost of the surgical stress.
Methods: We undertook a narrative review of the current literature on surgical prehabilitation and discussed the current evidence of preoperative interventions before cancer surgery in order to increase physiological reserve before surgery and accelerate postoperative recovery.
Results: Published data indicate the positive impact of prehabilitation on postoperative functional capacity and return to daily activities. However, the current evidence on the impact on short- and long-term clinical outcome is limited, and more research needs to be conducted.
Conclusion: Preliminary findings indicate that a group of interventions such as exercise, nutrition and anxiety reduction in the preoperative period can complement the enhanced recovery program and facilitate the return to baseline activities of daily living. It is not clear at this stage whether the preoperative increase in functional capacity mitigates the burden of postoperative morbidities and subsequent cancer therapies. Therefore, more research is warranted.
Advances in diagnostic procedures, surgical technology and perioperative care have improved safety and patient outcomes following surgical resection of cancer [Citation1]. Nevertheless, mortality and morbidity rates of major cancer surgical resection range between 4% and 10% [Citation2], and 20% and 60% [Citation3], respectively. Postoperative complications prolong hospital stay, readmissions and costs, have significant impact on patient functioning and quality of life, and may have long-term implications on mortality [Citation4].
The impact of the stress of cancer and surgery is notable during the recovery period, and is characterized by fatigue, decrease in appetite, pain, reduced mobilization and decrease in mental concentration. When the impact of abdominal surgery is evaluated using measures of functional capacity, only 30% of elderly people had recovered to preoperative levels at eight weeks after surgery and 50% after six months [Citation5]. Poor preoperative physical performance has been shown to increase the number of postoperative complications [Citation6,Citation7] and the risk of mortality [Citation8]. As the number of cancer patients increase with age, one has to consider the aging process itself which is associated with deconditioning, changes in body composition and function, loss of muscle mass and strength (sarcopenia), demineralization of bone, loss of aerobic capacity, loss of vasomotor stability and changes in respiratory function. Together, these phenomena contribute to a loss of functional capacity or the ability to perform tasks of daily life, thus impacting on the overall quality of life [Citation9]. Cancer prehabilitation has been defined as a ‘process on the cancer continuum of care that occurs between the time of cancer diagnosis and the beginning of acute treatment and includes physical and psychological assessments that establish a baseline functional level, identify impairments, and provide interventions that promote physical and psychological health to reduce the incidence and/or severity of future impairments’ [Citation10].
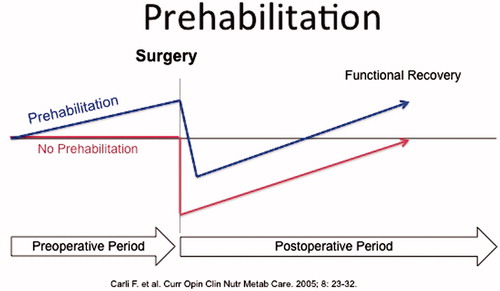
Although enhanced recovery after surgery (ERAS) programs have concentrated their efforts in optimizing the intra- and postoperative care, the preoperative period has received limited attention. Traditionally, efforts have been made to improve the recovery process by intervening once the patient has left the hospital. However, the postoperative time may not be the most appropriate time for cancer patients because many of them are concerned about interfering with the healing process, and some may be depressed and anxious as they wait for the result of the tumor pathology, which could imply additional treatments for the underlying condition. As many cancer patients are old with several comorbidities besides physical inactivity, at nutritional risk and not in appropriate health to sustain the stress of tumor resection, it would make sense to augment functional capacity through, for instance physical, nutritional and mental conditioning in anticipation of surgery. Topp [Citation11] and Ditmyer [Citation12] have proposed that, by applying a presurgical exercise program in patients, to improve functional ability before a stressor such as surgery, postoperative recovery would occur more rapidly compared to patients who remain inactive through surgical admission (). The present narrative review has been written with the intention of the authors to convey concepts of prehabilitation within the context of the ERAS program.
Methods
The authors met in December 2015 and the topics for inclusion were agreed upon and allocated. The principal literature search utilized MEDLINE, Embase and Cochrane databases to identify contributions related to the topic published between January 1999 and May 2015. Medical Subject Headings (MeSH) terms were used, as were accompanying entry terms for the patient group, interventions and outcomes. Keywords included exercise, perioperative nutrition, prehabilitation and functional outcome. All eligible articles were checked for other relevant studies. Conference proceedings were not searched. Based on the literature search, titles and abstracts were screened to identify reviews, case series, non-randomized studies, randomized control studies, meta-analyses and systematic reviews that were considered for each individual topic.
Results
Most human studies on prehabilitation have addressed the impact of physical activity/exercise on postoperative outcome following surgery, and with particular emphasis on orthopedic (hip and knee arthroplasty) and, more recently, cardiac, vascular and abdominal surgery. Three systematic reviews of fair to good methodological quality included 12, 15 and eight studies, respectively [Citation13–15]. The first review [Citation13] showed that preoperative exercise therapy can be effective for reducing postoperative complication rate and accelerating discharge from hospital in patients undergoing cardiac and abdominal surgery. The second [Citation14] examined 15 studies and concluded that total body prehabilitation improved postoperative pain, length of stay and physical function, but was not consistently effective in improving health-related quality of life or aerobic fitness. More recently, another systematic review of eight studies [Citation15] reported physiologic improvement with preoperative exercise, but with limited postoperative clinical benefits. Some aspects of the interventions used in these studies analyzed were not clearly presented; in fact, the exercise was not always structured, and the adherence to either low- or high-intensity exercise was not systematically reported. Although some degree of physiologic improvement during the preoperative period was shown by most of the studies, this change did not consistently translate into improved clinical outcomes. One example is a randomized controlled trial (RCT) [Citation16] in 112 patients undergoing colorectal surgery, who received a home-based program, which was either a sham intervention (control group with basic recommendation to walk daily and perform breathing exercises), or a home-based high-intensity training program (consisting of both aerobic and resistance exercise). Patients in the control group performed better than those who engaged in intense exercise of whom a large proportion deteriorated in functional walking capacity (measure of functional exercise capacity) during the presurgical period. Compliance was recorded at a mere 16%, indicating that the prescribed exercise regimen could not be maintained. Predictors of poor surgical outcome included presurgical deterioration, age greater than 75 years and high levels of anxiety. These results support the notion that an intervention based on intense exercise alone may not be sufficient to enhance functional capacity if other factors such as nutrition, anxiety and optimized perioperative care were not taken into consideration.
Recent reports have investigated nutritional and psychological interventions in combination with exercise. A pilot study [Citation17] followed by a RCT [Citation18], using a multimodal prehabilitation program composed of moderate intensity physical exercise, complemented by nutritional counseling, protein supplementation and anxiety reduction strategies, was conducted in 164 patients undergoing colorectal resection, and more than 80% of patients were able to return to preoperative functional capacity by eight weeks, compared with 40% of a control group.
Discussion
Data from reviewing the published literature on surgical prehabilitation of cancer patients highlight the potential impact that preoperative conditioning has on enhancing functional capacity before and after surgery. Structured exercise protocols represent the major component of the prehabilitation program, although other additional elements such as medical optimization, minimally invasive surgery and nutritional supplementation, would also contribute to improved clinical outcome. Regular exercise has been shown to decrease the incidence of ischemic heart disease, diabetes, hypertension, stroke and fractures in the elderly. In addition, physical training improves aerobic capacity, decreases sympathetic over-reactivity, improves insulin sensitivity and increases ratio of lean body mass to body fat.
It seems logical that, by increasing aerobic capacity and muscle, physiological reserve would be enhanced. The body would then be in better condition to compensate for the physical challenges of surgery and subsequently facilitate postoperative recuperation. Some components of a prehabilitation program may be of similar relevance to all types of surgery, such as aerobic and resistance exercise, smoking and alcohol cessation, anemia correction, ERAS, but specific interventions need to be tailored on a personal basis [Citation10]. The time interval for prehabilitation has been proposed to be between four and eight weeks, with shortest periods for patients with lung or abdominal cancer [Citation10]. In the following paragraphs, three core modalities of prehabilitation, exercise, nutrition supplementation and anxiety reduction will be discussed in light of available literature.
Exercise before surgery; effective doses, duration and adherence
A traditional approach to the preoperative timeframe is to encourage rest in order to best prepare the patient for their upcoming surgery. However, bed rest has deleterious effects on lean muscle mass, physical function, lower extremity strength/power, aerobic capacity and insulin sensitivity [Citation19–21]. Individuals with diminished insulin sensitivity are subject to a disproportionate aggravation of existing systemic low-grade inflammation during periods of physical inactivity [Citation22]. Exercise includes regular physical activity that is incorporated into a planned and structured program for the specific goal of improving fitness [Citation23]. Such a program results in a certain ‘dose’ of exercise that must be tailored to fit the desired outcomes for the patient. Prescribing exercise requires consideration of intensity (how physically challenging?), duration (how long?), frequency (how often?) and modality (how performed?). Although adhering to a lower ‘dose’ of physical activity (i.e. accumulating 30 minutes of physical activity over a day) has clear health benefits, higher ‘doses’ of exercise (i.e. 150 minutes of moderate to vigorous exercise per week) will result in greater improvements [Citation24]. In addition to cardiovascular exercise, it is equally important to consider resistance and flexibility training as important components of exercise prescription [Citation25,Citation26]. It must also be considered that exercise training is based on the principal of overload, thus, as the patient becomes accustomed to the intensity and adapts to the demands of the exercise performed, the intensity must be increased accordingly [Citation27] (). Importantly, there must be a balance between the ‘ideal’ intensity/amount of exercise, as proposed by existing guidelines/exercise principles and what is feasible for the patient.
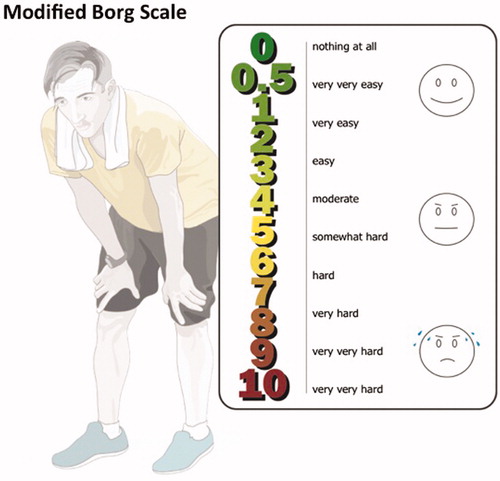
Figure 2. The Borg scale assesses the level of perceived exertion in response to exercise. The scale can be used to monitor improvement of fitness during an exercise program.
By participating in prehabilitation, it is possible for patients to increase their physical activity levels within the relatively short timeframe to meet current guideline recommendations [Citation28]. This is an important achievement, as the ‘dose’ of exercise determines the degree of physical improvements that are obtainable, thus most effectively preparing the patient for the stress of surgery [Citation29]. When prescribing an exercise program one should consider the patients’ needs/preferences for supervision (i.e. in hospital or home-based program with telephone follow-up), type of program (i.e. group classes or individual training) and modality (type of exercise). Other factors, such as parking and transportation, are also important to consider for patient adherence (Ferreira V. et al., unpubl. obs.). Unaccustomed exercise intensity or a program in which the patient does not feel confident can result in poor adherence. A patient-centered approach enhances adherence and maximizes the opportunity to achieve positive clinical outcomes in the presurgical timeframe [Citation30]. Regardless of the degree of supervision preferred by the patient, adequate oversight by a trained professional is imperative to ensure the exercises are being performed properly/safely and that there is sufficient progression to support the training process. Given the relatively condensed period in which prehabilitation is being performed, careful monitoring of the program is critical ().
Table 1. Physical activity and exercise goals in the presurgical period.
Optimize nutritional status before surgery: protein is the nutrient of focus
Protein requirements are elevated in stressed states, such as cancer, to account for added demands of hepatic acute phase proteins synthesis, and the synthesis of proteins involved in immune function and wound healing [Citation31]. Although optimal protein and amino acid intakes for cancer is unknown, non-surgical nutrition oncology guidelines on enteral nutrition suggest that cancer patients should consume at least 1.2–2.0 g protein/kg per day.
Dietary protein increases whole body protein synthesis by increasing systemic amino acid availability [Citation31]. Postexercise, the stimulatory effect that amino acids have on MPS appears to be enhanced [Citation32,Citation33]. In fact, protein ingestion postresistance exercise (performed until failure) has been found to stimulate rates of myofibrillar protein synthesis above fasting rates for 24 hours. This 24-hour period postresistance exercise has been dubbed the ‘anabolic window’ to reflect what appears to be a period of increased sensitivity of MPS to aminoacidemia.
Commercially available postexercise supplements are usually composed of whey, casein or soy. It is unclear which type of supplement has the greatest impact on performance in healthy or cancer patients. According to a recent report from the Food and Agriculture Organization of the United Nations (FAO), protein quality should be assessed using the Digestible Indispensable Amino Acid Score. Using this method to assess protein quality, milk proteins (including casein, milk protein concentrate, whole milk powder and whey protein isolates) are among the greatest sources of high quality protein [Citation34]. summarizes a practical example of a one-day food diary.
Table 2. Example of a standardized protein meal plan for prehabilitated patients.
The anxiety state of the surgical cancer patient
The presence of psychological distress, specifically anxiety and depression, is very common in patients with a cancer diagnosis. The treatment phase of the disease is associated with high rates of depression [Citation35]. Depression in this population has been shown to be associated with higher levels of pain, non-compliance with medical treatment, diminished immune response and carries an elevated risk of mortality [Citation36,Citation37]. In the context of surgery, psychological distress has been shown to impact on wound healing, postoperative pain relief, longer hospital stays and more functional limitations [Citation38].
Psychological distress at baseline has been identified as an important factor in influencing functional capacity during the pre- and postoperative periods. In over 100 patients enrolled in a study on the impact of prehabilitation in patients scheduled for colorectal cancer resection, high anxiety was reported in 42% of patients at baseline and the presence of depression was reported in 32% (Bernstein M., pers. comm.). When examining the differences between individuals who completed the prehabilitation program with those who dropped out, depression scores on the Hospital Anxiety Depression Scale (HADS) at baseline were significantly higher in the latter ones. A predictor of greater improvement in functional walking capacity during the preoperative period was the belief that fitness level aids recovery; high anxiety at baseline was also a significant predictor of poor recovery [Citation39]. These findings suggest that despite improvements made during the preoperative period, highly anxious individuals are still at risk for poor recovery.
High rates of anxiety and depression in the cancer population, as well as the negative effects of psychological distress on mental and physical wellbeing, have led to the study of whether preoperative psychological interventions can effectively reduce psychological distress in cancer patients. Evidence supporting the role for ‘psychological prehabilitation’ before surgery comes from RCTs in breast, colon and prostate cancer patients. Interventions implemented prior to surgery, such as relaxation techniques (deep breathing, progressive muscle relaxation and meditation), guided imagery and/or problem-solving and coping strategies, have been shown to improve quality of life [Citation40], reduce anxiety and depression [Citation40,Citation41], reduce pain severity and fatigue [Citation42].
There also exists evidence supporting a role for psychological prehabilitation in improving physical functioning and ability to perform ambulation and rehabilitation tasks. Parker and colleagues examined the effects of a presurgical stress management intervention in men with prostate cancer undergoing robotic prostatectomy and found less mood disturbance and higher quality of life [Citation41]. Williams and colleagues [Citation43] found that women undergoing mastectomy or hysterectomy who received a preoperative intervention meant to prepare them for surgery were better able to perform ambulation and rehabilitation tasks and self-care activities one month after surgery when compared to women not receiving the intervention [Citation43]. Although psychological prehabilitation may be an effective way to improve physical functioning in cancer patients, little research has been done to test this empirically. Studies of psychological prehabilitation have focused primarily on reducing psychological distress, attenuating immune suppression and improving surgical outcomes and have only measured physical functioning as a secondary outcome using self-report methods.
Critical appraisal of the published literature
We have discussed in this review the concept of surgical prehabilitation applied in the period between diagnosis and surgery in order to have a maximal impact on postoperative outcome.
With the increasing number of patients with comorbidities as a result of lifestyle and aging, it is necessary to integrate prehabilitation with the elements of the ERAS program. A multimodal approach with effective interventions during the preoperative period can include physical activity, nutrition, anti-anxiety strategies, correction of anemia, and alcohol and smoking cessation among others. Most of the studies reviewed included a limited number of patients scheduled for elective cancer surgery, and exercise was the most used intervention. Lately, nutrition and relaxation interventions have been added. Most of the studies indicating the positive effect of prehabilitation on postoperative functional capacity and return to daily activities and some studies indicated pain relief. Nevertheless, it remains to be seen whether prehabilitation can be applied in most of the surgical specialties, and whether perioperative outcomes can be improved. Therefore, the following research questions should be addressed in future studies: (1) What is the role of different types of exercise, the importance of nutrition optimization and psychological stress reduction in order to increase physiological reserve in anticipation of what could be the treatment, either surgery and/or chemotherapy and/or radiotherapy? and (2) What is the effectiveness of single and multiple modalities, their cost-effectiveness and the short- and long-term impact on clinical outcomes such as length of stay, hospital readmissions, emergency department visits, perioperative complications, oncologic burden and adherence to adjuvant treatment.
In conclusion, the preoperative period may be an opportune time to increase physical, mental and nutritional reserve in anticipation of surgery. Interventions like exercise, optimization of nutrition intake might be introduced in the preoperative period to mitigate the stress response and other side effects caused by surgery, thus potentially improving postoperative outcomes.
The increasing interest in surgical prehabilitation for surgical cancer patients stems from growing, however limited, evidence that such multidisciplinary programs can address modifiable risk factors that may impact treatment outcomes. Additionally, in the patient perspective, prehabilitation shows promising effects on preoperative functional capacity in anticipation of surgery, and with more research could mitigate the pathophysiological burden associated with cancer and surgical stress, thus accelerating the recovery process.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Fearon KC, Jenkins JT, Carli F, et al. Patient optimization for gastrointestinal cancer surgery. Br J Surg. 2013;100:15–27.
- Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364:2128–2137.
- Lucas DJ, Pawlik TM. Quality improvement in gastrointestinal surgical oncology with American College of Surgeons National Surgical Quality Improvement Program. Surgery. 2014;155:593–601.
- Khuri SF, Henderson WG, DePalma RG, et al. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg. 2005;242:326–341. discussion 41-3.
- Lawrence VA, Hazuda HP, Cornell JE, et al. Functional independence after major abdominal surgery in the elderly. J Am Coll Surg. 2004;199:762–772.
- Snowden CP, Prentis JM, Anderson HL, et al. Submaximal cardiopulmonary exercise testing predicts complications and hospital length of stay in patients undergoing major elective surgery. Ann Surg. 2010;251:535–541.
- Older P, Smith R, Hall A, et al. Preoperative cardiopulmonary risk assessment by cardiopulmonary exercise testing. Crit Care Resusc. 2000;2:198–208.
- Wilson RJ, Davies S, Yates D, et al. Impaired functional capacity is associated with all-cause mortality after major elective intra-abdominal surgery. Br J Anaesth. 2010;105:297–303.
- Varadhan KK, Neal KR, Dejong CH, et al. The enhanced recovery after surgery (ERAS) pathway for patients undergoing major elective open colorectal surgery: a meta-analysis of randomized controlled trials. Clin Nutrit (Edinburgh, Scotland). 2010;29:434–440.
- Silver JK, Baima J, Mayer RS. Impairment-driven cancer rehabilitation: an essential component of quality care and survivorship. CA Cancer J Clin. 2013;63:295–317.
- Topp R, Ditmyer M, King K, et al. The effect of bed rest and potential of prehabilitation on patients in the intensive care unit. AACN Clin Issues. 2002;13:263–276.
- Ditmyer MM, Topp R, Pifer M. Prehabilitation in preparation for orthopaedic surgery. Orthop Nurs. 2002;21:43–51.
- Valkenet K, van de Port IG, Dronkers JJ, et al. The effects of preoperative exercise therapy on postoperative outcome: a systematic review. Clin Rehabil. 2011;25:99–111.
- Santa Mina D, Clarke H, Ritvo P, et al. Effect of total-body prehabilitation on postoperative outcomes: a systematic review and meta-analysis. Physiotherapy. 2014;100:196–207.
- Lemanu DP, Singh PP, MacCormick AD, et al. Effect of preoperative exercise on cardiorespiratory function and recovery after surgery: a systematic review. World J Surg. 2013;37:711–720.
- Carli F, Charlebois P, Stein B, et al. Randomized clinical trial of prehabilitation in colorectal surgery. Br J Surg. 2010;97:1187–1197.
- Li C, Carli F, Lee L, et al. Impact of a trimodal prehabilitation program on functional recovery after colorectal cancer surgery: a pilot study. Surg Endosc. 2013;27:1072–1082.
- Gillis C, Li C, Lee L, et al. Prehabilitation versus rehabilitation: a randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology. 2014;121:937–947.
- Coker RH, Hays NP, Williams RH, et al. Bed rest promotes reductions in walking speed, functional parameters, and aerobic fitness in older, healthy adults. J Gerontol A Biol Sci Med Sci. 2015;70:91–96.
- Kortebein P, Symons TB, Ferrando A, et al. Functional impact of 10 days of bed rest in healthy older adults. J Gerontol A Biol Sci Med Sci. 2008;63:1076–1081.
- Hamburg NM, McMackin CJ, Huang AL, et al. Physical inactivity rapidly induces insulin resistance and microvascular dysfunction in healthy volunteers. Arterioscler Thromb Vasc Biol. 2007;27:2650–2656.
- Hojbjerre L, Sonne MP, Alibegovic AC, et al. Impact of physical inactivity on adipose tissue low-grade inflammation in first-degree relatives of type 2 diabetic patients. Diabetes Care. 2011;34:2265–2272.
- Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100:126–131.
- Haskell WL. J.B. Wolffe Memorial Lecture. Health consequences of physical activity: understanding and challenges regarding dose-response. Med Sci Sports Exerc. 1994;26:649–660.
- Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409–1426.
- Brown JC, Schmitz KH. The prescription or proscription of exercise in colorectal cancer care. Med Sci Sports Exerc. 2014;46:2202–2209.
- Garber CE, Blissmer B, Deschenes MR, American College of Sports Medicine position stand, et al. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334–1359.
- Chen BP, Awasthi R, Sweet SN, et al. Four-week prehabilitation program is sufficient to modify exercise behaviors and improve preoperative functional walking capacity in patients with colorectal cancer. Support Care Cancer. 2017;25:33–40.
- Bruns ER, van den Heuvel B, Buskens CJ, van Duijvendijk P, Festen S, Wassenaar EB, et al. The effects of physical prehabilitation in elderly patients undergoing colorectal surgery: a systematic review. Colorectal Dis. 2016;18:O267–O277.
- Butterworth SW. Influencing patient adherence to treatment guidelines. J Manag Care Pharm. 2008;14:21–24.
- Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutrit. 2006;84:475–482.
- Biolo G, Tipton KD, Klein S, et al. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol. 1997;273:E122–E129.
- Moore DR, Tang JE, Burd NA, et al. Differential stimulation of myofibrillar and sarcoplasmic protein synthesis with protein ingestion at rest and after resistance exercise. J Physiol. 2009;587:897–904.
- Wolfe RR. Update on protein intake: importance of milk proteins for health status of the elderly. Nutrit Rev. 2015;73(Suppl 1):41–47.
- Tsimopoulou I, Pasquali S, Howard R, et al. Psychological prehabilitation before cancer surgery: a systematic review. Ann Surg Oncol. 2015;22:4117–4123.
- Kitagawa R, Yasui-Furukori N, Tsushima T, et al. Depression increases the length of hospitalization for patients undergoing thoracic surgery: a preliminary study. Psychosomatics. 2011;52:428–432.
- DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160:2101–2107.
- Rosenberger PH, Jokl P, Ickovics J. Psychosocial factors and surgical outcomes: an evidence-based literature review. J Am Acad Orthop Surg. 2006;14:397–405.
- Mayo NE, Feldman L, Scott S, et al. Impact of preoperative change in physical function on postoperative recovery: argument supporting prehabilitation for colorectal surgery. Surgery. 2011;150:505–514.
- Garssen B, Boomsma MF, Meezenbroek Ede J, et al. Stress management training for breast cancer surgery patients. Psychooncology. 2013;22:572–580.
- Parker PA, Pettaway CA, Babaian RJ, et al. The effects of a presurgical stress management intervention for men with prostate cancer undergoing radical prostatectomy. J Clin Oncol. 2009;27:3169–3176.
- Larson MR, Duberstein PR, Talbot NL, et al. A presurgical psychosocial intervention for breast cancer patients. psychological distress and the immune response. J Psychosom Res. 2000;48:187–194.
- Williams PD, Valderrama DM, Gloria MD, et al. Effects of preparation for mastectomy/hysterectomy on women's post-operative self-care behaviors. Int J Nurs Stud. 1988;25:191–206.