Abstract
Background: The purpose of this study was to examine visual outcome, endocrine function and tumor control in a prospective cohort of craniopharyngioma patients, treated with fractionated stereotactic radiation therapy (FSRT).
Material and methods: Sixteen adult patients with craniopharyngiomas were eligible for analysis. They were treated with linear accelerator-based FSRT during 1999–2015. In all cases, diagnosis was confirmed by histological analysis. The prescription dose to the tumor was 54 Gy (median, range 48–54) in 1.8 or 2.0 Gy per fraction, and the maximum radiation dose to the optic nerves and chiasm was 54.2 Gy (median, range 48.6–60.0) for the cohort. Serial ophthalmological and endocrine evaluations and magnetic resonance imaging (MRI) scans were performed at regular intervals. Median follow-up was 3.3 years (range 1.1–14.1), 3.7 years (range 0.8–15.2), and 3.6 years (range 0.7–13.1) for visual outcome, endocrine function, and tumor control, respectively.
Results: Visual acuity impairment was present in 10 patients (62.5%) and visual field defects were present in 12 patients (75%) before FSRT. One patient developed radiation-induced optic neuropathy at seven years after FSRT. Thirteen of 16 patients (81.3%) had pituitary deficiency before FSRT, and did not develop further pituitary deficiency after FSRT. Mean tumor volume pre-FSRT was 2.72 cm3 (range 0.20–9.90) and post-FSRT 1.2 cm3 (range 0.00–13.10). Tumor control rate was 81.3% at two, five, and 10 years after FSRT.
Conclusions: FSRT was relatively safe in this prospective cohort of craniopharyngiomas, with only one case of radiation-induced optic neuropathy and no case of new endocrinopathy. Tumor control rate was acceptable.
Craniopharyngiomas are rare intracranial tumors, some of them presenting during childhood or adolescence, with persistence into adulthood. However, almost half of the patients are adults at presentation [Citation1,Citation2]. They account for approximately 2% of all intracranial neoplasms [Citation1]. They are usually benign epithelial tumors believed to originate from remnants of the Rathke’s pouch, and are either localized in the sellar or suprasellar region and can be cystic or solid, or both [Citation3]. Presenting symptoms include visual loss and endocrine disturbances [Citation2,Citation4]. Initial treatment is most often surgical [Citation2,Citation5,Citation6]. However, despite their benign histology, they often recur after surgery and can cause significant morbidity [Citation1,Citation5,Citation6].
Stereotactic radiosurgery (SRS) and fractionated stereotactic radiation therapy (FSRT) are highly precise image-guided radiation therapies primarily used for certain benign and malignant intracranial tumors. SRS and FSRT can either be used as a primary treatment of tumors, which are difficult to manage surgically, or as an adjuvant treatment to surgery, for residual or recurrent tumor [Citation7]. FSRT can be used to deliver large doses using high precision radiotherapy, and has the biological advantage of fractionation, with the aim of maximizing tumor control while minimizing side effects imposed on nearby eloquent structures [Citation8,Citation9].
There have been several published studies of the results of FSRT for craniopharyngiomas, consisting of smaller [Citation10–12] or larger cohorts [Citation3,Citation4,Citation13,Citation14]. These studies have consistently reported favorable tumor control, but conflicting results in terms of risks and side effects concerning vision and endocrine function after FSRT [Citation3,Citation4,Citation10–14]. We now report our findings in a prospective cohort of 16 consecutively treated adult patients with craniopharyngiomas, treated over a 17-year period with FSRT, regarding visual outcome, endocrine function, and tumor control.
Patients and methods
FSRT for benign anterior skull base tumors, including craniopharyngiomas, has been offered as a routine treatment at Rigshospitalet, Copenhagen, Denmark since 1999. All patients were recruited consecutively and prospectively, during the period January 1999 – December 2015. Diagnosis was confirmed by histological analysis after surgery in all cases. Residual or recurrent tumors were diagnosed by follow-up magnetic resonance imaging (MRI) scans. The present study contains only adult patients treated at our institution using FSRT, as children with craniopharyngiomas are usually referred to proton beam therapy at an external institution [Citation15]. A total of 17 adult patients with craniopharyngiomas were treated with FSRT during the study period. One patient, living abroad, was lost to follow-up, leaving 16 patients included in this study. There were seven females and nine males. Mean age at the time of FSRT was 51 years (range 17–71) ().
Table 1. Patients, tumor characteristics and surgical treatment.
Tumor characteristics and surgical treatment
The tumor and organs at risk were manually defined using computed tomography (CT) and MRI scans in the clinical treatment planning software by a radiation oncologist and radiologist at the Department of Radiation Oncology. Mean tumor volume at the time of FSRT was 2.72 cm3 (range 0.20–9.90). Tumor localization and extension were defined from MRI scans. Seven (44%) tumors were intrasellar, five (31%) were suprasellar and four (25%) were both intrasellar and suprasellar.
Solid and cystic components of tumor were identified on MRI. Nine patients (56.3%) had solid tumor, six (37.5%) patients had both cystic and solid tumor and one patient (6.2%) had cystic tumor. All patients had undergone a median of two operations (range 1–4), prior to FSRT, with a median time from latest operation until FSRT of 20.5 months (range 5–118). The indications for FSRT were progression of residual tumor after subtotal resection in 10 (62.5%) patients and tumor recurrence after macroradical resection in six (37.5%) patients. Furthermore, three patients underwent repeat surgical resection for residual tumor progression, at 10.7, 11.8 and 13.7 months after FSRT, respectively (). By comparison, the average number of operations for craniopharyngiomas performed was 12 per year at our center, which is a national referral center for the treatment of craniopharyngiomas. FSRT for craniopharyngiomas, is in turn, exclusively offered nationwide at our institution.
Histopathology
A diagnosis of craniopharyngiomas was verified in all cases by routine histology. The histology was reevaluated by one of the authors, a neuropathologist (HB), for the purpose of this study. Fourteen (87.5%) of the tumors were of the adamantinomatous histological type, one (6.3%) was of the papillary histological type, and histological type could not be verified in one case (6.3%) of an exclusively cystic tumor.
Fractionated stereotactic radiation therapy
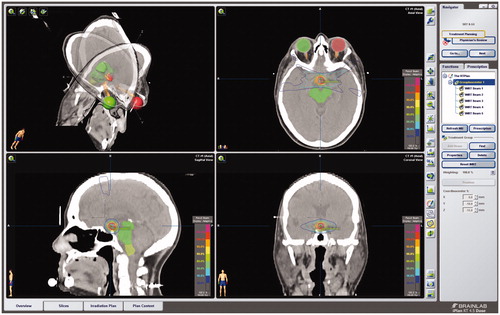
In all cases, linear accelerators were used to deliver the stereotactic irradiation treatments; a dedicated stereotactic accelerator (Clinac 600SR, Varian Medical Systems, Palo Alto, CA, USA) was used 1999–2008, and was thereafter replaced by three NovalisTx© (Varian Medical Systems and BrainLab, Munich, Germany). Fixed circular collimators, with 0.5–3.5 cm diameter were used for the first patients and since August 2000, collimation and field shaping has been provided using micromultileaf collimators. The treatment planning was performed using a system dedicated for stereotactic radiotherapy (XKnife, RSA, USA, and BrainScan and iPlan, BrainLab and since 2012 Varian Eclipse). An individual aquaplastic mask of the head was made during the planning process and was used for all the treatments. A fusion of MRI and CT scans was used as a visual treatment plan, whereby the gross target volume was estimated. The target volume was defined and treated without setup margins. The eye balls, optic chiasm, nerves and tracts and brainstem were outlined and defined as organs at risk. The prescription dose of 54 Gy was given to the 90% isodose contour, and the 90% isodose contour was encompassing the target volume. Prescribed tumor radiation doses for 14 patients were 1.8 Gy per fraction given in 30 fractions and for two patients were 2.0 Gy per fraction given in 24 and 27 fractions, respectively. Four patients received treatment with intensity modulated radiation therapy (IMRT) and 12 patients received treatment using dynamic conformal arc radiation therapy (DCART). For an example of a treatment plan, see .
Figure 1. Example dose plan in iPlan (BrainLab) for IMRT of a craniopharyngioma, with coronal, axial and sagittal CT overlaid on MRI with gadolinium (Gd) enhancement. The target volume was defined and treated without setup margins. The eye balls, optic chiasm, nerves and tracts, and brainstem were outlined and defined as organs at risk. The prescription dose of 54 Gy was prescribed to the 90% isodose contour, and the 90% isodose contour was encompassing the target volume.
Visual outcome and optic radiation
Ophthalmological examination was performed at baseline and at nine months, two years, 3.5 years, five years, seven years and up to at least 10 years after treatment. Visual acuity was measured with a Snellen eye chart and an improvement in visual acuity was defined as a gain of 0.2 or more, and worsening as a loss of 0.2 or more. Visual fields were divided into 24 sectors, representing the central 30° for each eye and quantified with Campimetry, Goldmann dynamic perimetry and Octopus static perimetry [Citation16].
The visual outcome was defined as radiation-induced optic neuropathy if visual acuity dropped from greater than 0.20–0.20 or less on one or both eyes and/or if the visual fields decreased by 1/24 fields or more on one or both eyes, in the absence of tumor growth.
The combined optic structures (optic nerves, chiasm and tracts) were outlined by BrainScan or BrainLab autocontouring function. The presence or absence of direct contact between tumor and anterior optic pathways was determined from the three-dimensional (3D) dose plan MRI scans. Optic dosimetry data were extracted from the dose plan records. Only the maximum dose to the combined optic structure was considered, extracting the maximum of the dose delivered to the optic chiasm, optic nerves and tracts. Median of the maximum COS dose was 54.2 (range 48.6–60.0) Gy and tumor was in direct contact with the anterior optic pathways in 13 patients (81.3%).
Pituitary and hypothalamic function
The patients underwent at least annual assessment of anterior pituitary function at the department of medical endocrinology between 9a.m. and 11a.m., after an overnight fast. The patients rested 15–30 minutes prior to testing, after inserting an indwelling catheter in a large forearm vein, and baseline samples were drawn for analysis of thyroid stimulating hormone (TSH), free thyroxine (fT4), luteinizing hormone (LH), follicle stimulating hormone (FSH), total testosterone (in men), estradiol (in women), total cortisol, growth hormone, insulin-like growth factor-I and insulin-like growth factor binding protein-3. An insulin tolerance test was performed in all patients except for those with overt contraindications such as epilepsy or ischemic vascular disease, where an arginine-growth-hormone-releasing-hormone test was chosen using body mass index related cutoffs for growth hormone as previously described [Citation17].
Pituitary deficiency was defined by biochemical deficiency. Thus, deficiency of the hypothalamo-pituitary-adrenal axis efficiency was diagnosed if baseline or stimulated (Synacthen® or insulin tolerance test) cortisol levels did not reach 500 nmol/l [Citation18]. Growth hormone function was screened by serum IGF-I and IGFBP-3. TSH deficiency was defined when fT4 was low (below or in the low normal range) with an inappropriately low TSH. Hypogonadotrophic hypogonadism in post-menopausal women was defined as inappropriately low gonadotrophins for age; in pre-menopausal women as the presence of amenorrhea or oligomenorrhoea, associated with persistently low estradiol and inappropriately low gonadotrophins; and in men as a low serum total testosterone (<10 mmol/l) associated with inappropriately low LH. Panhypopituitarism was defined as deficiency of all four axes. Replacement therapy was initiated when clinical signs and/or biochemistry indicated the need. Prolactin function was not routinely measured, and is not included in this study. It was noted whether patients had diabetes insipidus requiring medical therapy, or other abnormality of hypothalamic function. The pituitary and hypothalamic regions, were not defined as organs at risk (OAR), at the time of treatment.
Tumor control measures
MRI neuroimaging for dose planning was performed prior to FSRT, and at 9 months, 2, 3.5, 5, 7 years and up to at least 10 years, after treatment. In all cases T1-weighted MRI images of the head in the three planes were performed, after application of gadolinium contrast. Tumor extent was determined from pre-treatment and the latest post-treatment contrast T1 weighted MRI images. Both solid and cystic parts of tumor were included. Tumor volume was calculated using 3D volumetric assessment with the BrainLab Brainscan or iPlan or the Varian Eclipse treatment planning software. Tumor response was evaluated by fusion of pre- and post-FSRT MRI and CT scans of the treatment plans, with the gross target volume as reference. To assess the significance of change in tumor volume after FSRT, the Wilcoxon’s signed ranks test, was used. Change in tumor volume after FSRT was defined as a complete response when there was an absence of tumor, partial response when tumor volume was diminished by ≥25% and stable disease when tumor volume was diminished by <25% with no sign of progression. Tumor progression was assessed as any increase of tumor volume by ≥25%. Tumor control was defined as a complete response, partial response, or stable size. Tumor control rates were assessed using the Kaplan-Meier method.
Results
Visual function
Median follow-up after FSRT was 3.3 years (range 0.5–14.1). Mean visual acuity of the left eye was 0.72 (sd ± 0.39) pre- and 0.70 (sd ± 0.41) post-FSRT and of the right eye 0.83 (sd ± 0.29) pre- and 0.89 (sd ± 0.23) post-FSRT.
This included four patients who prior to FSRT had very poor visual acuity on one eye; one patient was almost blind following operation, with only finger counting response on the left eye, and three patients had a unilateral visual acuity of 0.10, 0.10 and 0.30, respectively, which were due to amblyopia, macular degeneration and cataract, and amblyopia and cataract, respectively. At the latest follow-up after FSRT, six patients (37.5%) with intact visual acuity before FSRT still had intact visual acuity after FSRT; seven patients (43.8%) with impaired visual acuity before FSRT, had unchanged impaired visual acuity after FSRT and one patient (6.3%) with impaired visual acuity before FSRT had improved visual acuity after FSRT. Two patients with impaired visual acuity before FSRT had worsened visual function following FSRT in the absence of tumor growth; one with a decrease in visual acuity, which did not drop below 0.20 and thus did not meet our criteria for radiation-induced optic neuropathy (); and one within our radiation-induced optic neuropathy criteria with visual acuity on the left eye dropping from 0.30 to 0.02, as well as dropping on the right eye from 0.70 to 0.50. The latter patient had a pre-FSRT history of amblyopia and cataract (see also above) and a recent cataract operation after FSRT, which failed to improve vision. A diagnosis of radiation-induced optic neuropathy was made by the ophthalmologists, seven years after FSRT. Additionally, this patient had a bitemporal field defect before FSRT, due to prior tumor compression, which remained unchanged after FSRT. Thus, one of 16 patients (6.3%) developed radiation-induced neuropathy, affecting visual acuity. The patient was 71 years at the time of FSRT and tumor volume was 1.95 cm3. The total tumor radiation dose was 54 Gy and maximal optic dose was 60 Gy, and there was direct tumor-optic contact.
Table 2. Visual function after FSRT.
Mean visual field defects, were on the left eye 7.94 (sd ± 6.12) pre- and 7.94 (sd ± 6.12) post-FSRT and on the right eye 6.38 (sd ± 5.24) pre- and 6.53 (sd ± 5.38) post-FSRT.
Visual fields were intact in four patients and remained intact after FSRT. Visual fields were impaired in 12 patients, and improved in one patient with a minor upper temporal quadrant defect and remained impaired in 11 patients after FSRT, including six with hemianopia, one with hemianopia with macular sparing, three with quadrantanopia and one with partial quadrantopia (). The visual field defects were caused by prior tumor compression of the optic pathways in 11 cases and by operation in one case (see also above).
Endocrine function
Median follow-up after FSRT was 3.7 years (range 0.8–15.2 years). Three patient (18.8%) had normal pituitary function, before and after FSRT, one patient (6.3%) had two deficient axes before and after FSRT, three patients (18.8%) had three deficient axes before and after FSRT, and nine patients (56.3%) had panhypopituitarism before and after FSRT, and in all cases the same axes were deficient before and after FSRT (). Thus, 12 of 16 (75%) had pituitary deficiency, at the time of FSRT; thereof 10 due to operation, two due to prior tumor compression, and one due to tumor compression and further deficiency due to operation (two axes pre- and four axes post-operatively). No patient developed a new pituitary deficiency after FSRT. All patients with hypopituitarism received substitution therapy of two or more hormones.
Table 3. Pituitary hormone deficiency.
Eleven patients (68.8%) had diabetes insipidus, requiring desmopressin treatment, before and after FSRT, and no additional case of diabetes insipidus occurred following FSRT. Additionally, three patients were obese, at the time of FSRT, and one of these patients developed type 2 diabetes 10 years after FSRT.
Tumor control
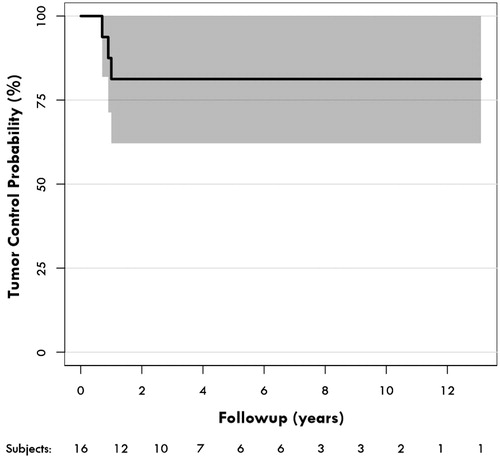
Median follow-up after FSRT was 3.7 years (range 0.7–13.1). Mean tumor volume pre-FSRT was 2.72 cm3 (range 0.20–9.90) and post-FSRT 1.28 cm3 (range 0.00–13.10), respectively, and this difference was significant (p = 0.015, Wilcoxon’s signed ranks test). Overall, tumor control was accomplished in 13 of 16 (81.3%) patients. Of the nine cases with solid tumors (56.3%), all remained solid after FSRT. Of the six cases of mixed cystic and solid tumors (37.5%), two remained cystic and solid and four were solid only, and the one cystic tumor (6.25%) remained cystic after FSRT. There were no new cyst formations in relation to FSRT. Nine patients (56.3%) had a complete tumor response and four patients (25%) had a partial response. Three patients (18.7%) had tumor progression, requiring reoperation at 10.7, 11.8 and 13.2 months, respectively. The three tumors which progressed, were all of the adamantinomatous histological type. Actuarial tumor control rates were accordingly 81.3% (95% CI 52.4–93.5) at two, five and 10 years after FSRT ().
Mortality
One patient died five years after FSRT at the age of 73 and one patient died eight years after FSRT at the age of 80, both from respiratory failure, presumed unrelated to the tumor disease or treatment.
Discussion
This study represents one of few prospective cohorts of visual outcome, endocrine function and tumor control after LINAC-based FSRT of craniopharyngiomas. The study contains only 16 patients over 17 years, at a large national neurosurgery and radiation oncology center, which reflects the rarity of this tumor disease and the fact that the primary treatment is surgical [Citation5,Citation6].
Most visual defects observed in the cohort were present at the time of FSRT and related to tumor compression. However, one of 16 patients at risk developed radiation-induced optic neuropathy, with new visual acuity deterioration. This patient received the highest maximum dose of 60 Gy to the optic system and there was direct tumor-optic contact. However, for the whole cohort, the median dose to the optic system was 54.2 and there was direct tumor-optic contact in the majority of cases, yet only one patient was affected. We recently reported a 10–13% occurrence of mostly mild to moderate radiation-induced optic neuropathy in a larger cohort of anterior skull base meningiomas and pituitary adenomas [Citation16], evaluated using the same methodology. The crude incidence of radiation-induced optic neuropathy of the present craniopharyngioma cohort is similar, though direct comparison is difficult with only one event and shorter follow-up in the present study [Citation16]. In our previous study [Citation16], the occurrence of radiation-induced optic neuropathy correlated with increasing age. The patient with a radiation-induced optic neuropathy was the oldest patient in the present study. Also, median tumor volume was considerably smaller in the present study, with consequently less irradiated volumes [Citation16]. The presence of pre-existing visual impairment did not appear to predispose patients to radiation-induced optic neuropathy in this cohort, in contrast to our previous study [Citation16]. Radiation-induced optic neuropathy occurred, as a late event, seven years after FSRT for the single patient in this study, in line with our previously published cohort [Citation16].
Several reports of FSRT-treated craniopharyngioma patients have described radiation-induced optic neuropathy. Hashizume et al. found one case of radiation-induced optic neuropathy in a cohort of 10 patients with craniopharyngiomas [Citation11]. Also, Minniti et al., in a prospective study of 39 patients, reported one patient with severe visual impairment prior to radiotherapy having further visual deterioration, due to the stereotactic radiation therapy [Citation13]. Finally, Harabi et al. in a long-term outcome study of 55 craniopharyngioma patients treated with FSRT, reported worsening vision in one patient, but it was not clear if this was due to radiation-induced optic neuropathy [Citation14].
In contrast, no radiation-induced optic neuropathies were observed after FSRT of craniopharyngiomas in a retrospective study by Selch et al. [Citation12], a study by Schulz-Ertner et al. of 26 patients [Citation4], and a subsequent study by the same group and extended cohort of 40 patients [Citation3]. Finally, Kanesaka et al., in a retrospective study, also did not observe visual deterioration in any of their 16 patients [Citation10].
The pituitary gland is known to be particularly sensitive to radiation in a dose dependent manner [Citation19]. However, at the time of FSRT in the present study, pituitary deficiency of 2–4 axes was already present in 12 of the 16 patients (75%). Thus, most often the pituitary deficiency was a result of tumor and/or prior operation. Notably, with macroradical tumor resection, it often not possible to spare the pituitary stalk [Citation4]. There was no sign of radiation-induced pituitary deficiency in this study. Combs et al. reported new pituitary deficiencies in two of 40 craniopharyngioma patients treated [Citation3], and Minniti et al. reported that 42% of their 39 craniopharyngioma patients developed new or worsening pituitary deficiency after FSRT [Citation13]. On the contrary, Selch et al. in their retrospective study of 16 craniopharyngioma patients reported no endocrinopathies resulting from FSRT [Citation12]. Also, Kanesaka et al. did not observe worsening in hormonal function of any of their 16 craniopharyngioma patients [Citation10]. Finally, in the study by Harabi et al., none of the 55 patients in the study developed new endocrinopathies after FSRT [Citation14].
In our study, diabetes insipidus was present in 11 of 16 patients at the time of FSRT, and persisted at latest follow-up after FSRT, but no new cases after FSRT were observed, in keeping with previous reports [Citation4,Citation13].
As pituitary and hypothalamic regions were not defined as OAR during dose planning and no new endocrinopathies after FSRT were detected, it cannot be concluded, based on the present study, that the pituitary gland and hypothalamus should be defined as OAR during dose planning, particularly in case pituitary function is already extinct.
It is not clear why some studies revealed visual complications or endocrinopathies after FSRT, and some did not, as most of the studies used a similar radiation dose [Citation3,Citation4,Citation12–14], although study design and follow-up time varied [Citation3,Citation4,Citation10–14].
At a median follow-up of 3.6 years tumor control seemed acceptable at 81.3%, accomplished in 13 of the 16 patients, and three patients having recurrent tumor around one year after FSRT. It was not clear why these three tumors progressed, as they were similar in size and received a similar radiation dose as other tumors in this cohort. These three tumors were of the adamantinomatous histology type, which is the most common, representing around 90% of all craniopharyngiomas. The less common papillary histological type, however, may be more radiosensitive [Citation20] and have a lower recurrence risk [Citation1,Citation21]. Selch et al. in their study of 16 craniopharyngioma patients reported a three-year tumor control rate of 93% after FSRT. Schulz-Ertner et al., in their cohort of 26 craniopharyngioma patients, reported a 100% tumor control rate at five and 10 years after treatment with FSRT [Citation4] and in their follow-up study of 40 patients, observed the same 100% five- and 10-year tumor control rate [Citation3]. Minniti et al. reported a progression-free survival of 97% at three and 92% at five years after FSRT in a cohort of 39 patients [Citation13]. Hashizumi et al. reported a 100% tumor control rate of 10 cases of craniopharyngiomas at a median follow-up of 25.5 months [Citation11]. Kanesaka et al. reported a three-year tumor control rate of 82.4% after FSRT of their 16 craniopharyngioma patients [Citation10]. Finally, Harabi et al., with their study of 55 FSRT-treated craniopharyngioma patients, reported a five- and a 10-year tumor control rate of 95.3% and 92.1%, respectively [Citation14].
Frequent cyst developments following SRS and radiation therapy have been described [Citation3,Citation4,Citation13,Citation22], but no new cyst formations were observed after FSRT in the present cohort.
In contrast to previous studies [Citation3,Citation4,Citation13,Citation14], there were no children in our cohort, as the policy in our institution is to refer children for proton beam therapy at an external institution. Proton beam therapy for craniopharyngiomas is also an effective treatment modality, with favorable long-term cystic and nodular tumor control rates, but can also be associated with visual complications and significant endocrinopathies [Citation15].
Also, single dose SRS for craniopharyngiomas, has proven effective in several studies, but also associated with side effects in some cases. Chiou et al. reported an overall tumor control rate of 87%, and no new endocrine dysfunction, but visual deterioration in two of 10 patients after radiosurgery [Citation22]. Chung et al. reported 31 patients with craniopharyngiomas treated with Gamma Knife radiosurgery, with an 87% tumor control and one case of visual deterioration, but no new endocrine abnormalities [Citation23].
To this end, while SRS and FSRT are both high precision radiation techniques, FSRT has the biological advantage of fractionation [Citation8,Citation16]. Given a similar tumor control rate, FSRT may therefore be preferable, as the required therapeutic dose for SRS treatment of craniopharyngiomas may be close to the maximal single dose of only 10–15 Gy tolerated by the optic system [Citation16]. Additionally, only cases with a margin of at least 3 mm between tumor and optic system, are considered suitable for SRS [Citation24].
In conclusion, in a cohort of 16 adult patients with craniopharyngiomas, treated with FSRT, there was an overall favorable tumor control rate and the therapy was relatively safe in terms of visual outcome and endocrine function. However, the incidence of craniopharyngiomas is low, and the majority of craniopharyngiomas are primarily managed surgically [Citation5,Citation6,Citation25], with SRS/FSRT often reserved for recurrent or progressive tumors [Citation25,Citation26]. Therefore, larger scale, possibly multi-center studies, or meta-analyses may be warranted. Also, the rarity of craniopharyngiomas, makes a case of centralizing FSRT treatment of this tumor disease further.
Ethical standards
The study was approved by the Danish Data Protection Agency and was conducted according to Danish legislation, and the Helsinki II declaration. Ethical committee approval is not required in Denmark for clinical audits.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Karavitaki N, Cudlip S, Adams CB, et al. Craniopharyngiomas. Endocr Rev. 2006;27:371–397.
- Trippel M, Nikkhah G. Stereotactic neurosurgical treatment options for craniopharyngioma. Front Endocrinol (Lausanne). 2012;3:63.
- Combs SE, Thilmann C, Huber PE, et al. Achievement of long-term local control in patients with craniopharyngiomas using high precision stereotactic radiotherapy. Cancer. 2007;109:2308–2314.
- Schulz-Ertner D, Frank C, Herfarth KK, et al. Fractionated stereotactic radiotherapy for craniopharyngiomas. Int J Radiat Oncol Biol Phys. 2002;54:1114–1120.
- Fahlbusch R, Honegger J, Paulus W, et al. Surgical treatment of craniopharyngiomas: experience with 168 patients. J Neurosurg. 1999;90:237–250.
- Yasargil MG, Curcic M, Kis M, et al. Total removal of craniopharyngiomas. Approaches and long-term results in 144 patients. J Neurosurg. 1990;73:3–11.
- Elhateer H, Muanza T, Roberge D, et al. Fractionated stereotactic radiotherapy in the treatment of pituitary macroadenomas. Curr Oncol. 2008;15:286–292.
- Roug S, Rasmussen AK, Juhler M, et al. Fractionated stereotactic radiotherapy in patients with acromegaly: an interim single-centre audit. Eur J Endocrinol. 2010;162:685–694.
- Nutting C, Brada M, Brazil L, et al. Radiotherapy in the treatment of benign meningioma of the skull base. J Neurosurg. 1999;90:823–827.
- Kanesaka N, Mikami R, Nakayama H, et al. Preliminary results of fractionated stereotactic radiotherapy after cyst drainage for craniopharyngioma in adults. Int J Radiat Oncol Biol Phys. 2012;82:1356–1360.
- Hashizume C, Mori Y, Kobayashi T, et al. Stereotactic radiotherapy using Novalis for craniopharyngioma adjacent to optic pathways. J Neurooncol. 2010;98:239–247.
- Selch MT, DeSalles AA, Wade M, et al. Initial clinical results of stereotactic radiotherapy for the treatment of craniopharyngiomas. Technol Cancer Res Treat. 2002;1:51–59.
- Minniti G, Saran F, Traish D, et al. Fractionated stereotactic conformal radiotherapy following conservative surgery in the control of craniopharyngiomas. Radiother Oncol. 2007;82:90–95.
- Harrabi SB, Adeberg S, Welzel T, et al. Long term results after fractionated stereotactic radiotherapy (FSRT) in patients with craniopharyngioma: maximal tumor control with minimal side effects. Radiat Oncol. 2014;9:203.
- Bishop AJ, Greenfield B, Mahajan A, et al. Proton beam therapy versus conformal photon radiation therapy for childhood craniopharyngioma: multi-institutional analysis of outcomes, cyst dynamics, and toxicity. Int J Radiat Oncol Biol Phys. 2014;90:354–361.
- Astradsson A, Wiencke AK, Munck af Rosenschold P, et al. Visual outcome after fractionated stereotactic radiation therapy of benign anterior skull base tumors. J Neurooncol. 2014;118:101–108.
- Klose M, Stochholm K, Janukonyte J, et al. Prevalence of posttraumatic growth hormone deficiency is highly dependent on the diagnostic set-up: results from The Danish National Study on Posttraumatic Hypopituitarism. J Clin Endocrinol Metab. 2014;99:101–110.
- Klose M, Lange M, Rasmussen AK, et al. Factors influencing the adrenocorticotropin test: role of contemporary cortisol assays, body composition, and oral contraceptive agents. J Clin Endocrinol Metab. 2007;92:1326–1333.
- Littley MD, Shalet SM, Beardwell CG, et al. Hypopituitarism following external radiotherapy for pituitary tumours in adults. Q J Med. 1989;70:145–160.
- Inoue HK, Nakamura M, Ono N, et al. Radiosensitive squamous cell craniopharyngioma: clinical and pathological comparison with the adamantinomatous type. Noshuyo Byori. 1993;10:27–31.
- Sartoretti-Schefer S, Wichmann W, Aguzzi A, et al. MR differentiation of adamantinous and squamous-papillary craniopharyngiomas. AJNR Am J Neuroradiol. 1997;18:77–87.
- Chiou SM, Lunsford LD, Niranjan A, et al. Stereotactic radiosurgery of residual or recurrent craniopharyngioma, after surgery, with or without radiation therapy. Neuro-oncology. 2001;3:159–166.
- Chung WY, Pan DH, Shiau CY, et al. Gamma knife radiosurgery for craniopharyngiomas. J Neurosurg. 2000;93(Suppl 3):47–56.
- Leber KA, Bergloff J, Pendl G. Dose-response tolerance of the visual pathways and cranial nerves of the cavernous sinus to stereotactic radiosurgery. J Neurosurg. 1998;88:43–50.
- Van Effenterre R, Boch AL. Craniopharyngioma in adults and children: a study of 122 surgical cases. J Neurosurg. 2002;97:3–11.
- Niranjan A, Kano H, Mathieu D, et al. Radiosurgery for craniopharyngioma. Int J Radiat Oncol Biol Phys. 2010;78:64–71.
- Ulfarsson E, Lindquist C, Roberts M, et al. Gamma knife radiosurgery for craniopharyngiomas: long-term results in the first Swedish patients. J Neurosurg. 2002;97:613–622.