Abstract
Background: Tamoxifen is a well established treatment for breast cancer, but its long-term effects on the incidence of secondary cancers are not fully evaluated.
Material and methods: We have studied 4128 postmenopausal patients with early stage breast cancer who were alive and free of breast cancer recurrence after two years of tamoxifen, and who were randomized to receive totally two or five years of therapy.
Results: Compared to patients randomized to two years of tamoxifen the incidence of contralateral breast cancer [hazard ratio (HR) 0.73; 95% CI 0.56–0.96] and of lung cancer (HR 0.45; 95% CI 0.27–0.77), especially squamous cell and small cell lung cancer, were reduced in the five-year group, and similar results were seen when restricting the analysis to the 10-year period after treatment stopped. An increased incidence of endometrial cancer was observed in the five-year group, but the excess risk decreased over time.
Conclusion: Further studies of the effects of tamoxifen on the risk of different histological types of lung cancer are needed.
Tamoxifen is a well established adjuvant treatment for early breast cancer which reduces breast cancer recurrence and mortality rates. It is also established that tamoxifen influences the risk of secondary cancer, for instance reduces the risk of contralateral breast cancer and increases the risk of endometrial cancer, but it is less clear how long these effects last after cessation of treatment [Citation1,Citation2]. Generally, we need more information on the long-term effects of tamoxifen on secondary cancer risks. We have now updated the large Swedish breast cancer trial comparing five with two years of tamoxifen [Citation3] with information on new cancers from the national cancer register and report the long-term effects of prolonged tamoxifen treatment on the incidence of second primary cancer.
Material and methods
The trial was planned and organized by the Swedish Breast Cancer Group and involved five regional breast cancer study organizations (Supplementary Table 1). During the period 1982–1992 a total of 4610 postmenopausal women younger than 75 years of age with early stage invasive breast cancer were entered into a randomized trial comparing five and two years of adjuvant tamoxifen. Twenty milligram daily doses were used at two centers and 40 mg daily doses at the remaining three centers. Of these women 4128 remained alive, had no recurrence, and no contralateral breast cancer two years after surgery and could thus contribute meaningful information to two-year (n = 2106) versus five-year (n = 2022) comparisons. Data on new cancers were obtained from the Swedish Cancer Registry. Mean age at treatment start was 62.7 years in the two-year group and 62.6 years in the five-year group.
To avoid the possible confounding effect of recurrence of breast cancer and subsequent treatment, each patient was considered to be at risk for the analyzed events from two years of the date of operation up to, but not beyond, that of any recurrence or contralateral breast cancer. Cox proportional hazards model, stratified by trial center, was used to obtain hazard ratios (HR), CIs, and p values. Cumulative incidence probabilities were estimated by use of the life-table method. Analyses were done by the intention-to-treat.
Ethical approval
The present study of long-terms effects of tamoxifen treatment has been carried out with the approval of the Regional Ethical Review Board in Linköping (Dnr 2011/317-31).
Results
The total number of patients with second primary cancers was 413 in the five-year group and 395 in the two-year group, and the HR was 1.01 (95% CI 0.88–1.16) (Supplementary Table 2). Three cancer diagnoses showed significant differences in incidence between treatment groups: contralateral breast cancer (p = 0.022) (), endometrial cancer (p = 0.0059) (), and lung cancer (p = 0.0038) (), but none of the other 30 cancer types analyzed.
Figure 1. Cumulative incidence for contralateral breast cancer (A), endometrial cancer (B) and lung cancer (C) among patients without prior recurrences or contralateral breast cancer randomly assigned to 2 years (n = 2106) or 5 years (n = 2022) of adjuvant tamoxifen therapy.
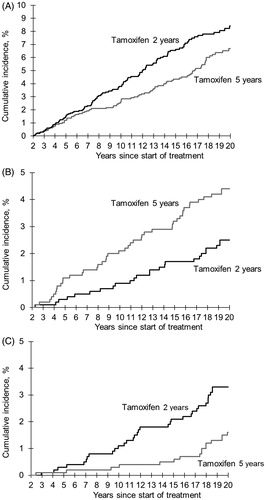
The incidence rate of new primary cancer in the contralateral breast was significantly lower in the five-year group as compared with the two-year group also after cessation of treatment (HR 0.71, 95% CI 0.52–0.97) (), and further division of the follow-up period suggests that the risk reduction may last up to 10 years post-treatment, but not thereafter (, Supplementary Table 3).
Figure 2. Hazard ratios (5 vs. 2 years of tamoxifen) for contralateral breast cancer (A), endometrial cancer (B) and lung cancer (C) among patients without prior recurrences or contralateral breast cancer randomly assigned to 2 years (n = 2106) or 5 years (n = 2022) of adjuvant tamoxifen therapy.
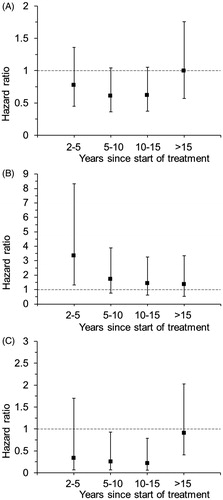
Table 1. Secondary cancer incidence without prior recurrence or contralateral breast cancer according to randomized treatment (5 vs. 2 years of tamoxifen) for the entire follow-up (>2 years after start of treatment), during treatment (2–5 years) and after treatment (>5 years).
The risk of endometrial cancer was significantly increased in the five-year group during treatment (HR 3.33, 95% CI 1.33–8.34) (), but the observed excess risk decreased substantially by time from treatment cessation (, Supplementary Table 3).
Prolonged tamoxifen treatment substantially reduced the incidence of lung cancers (HR 0.45, 95% CI 0.27–0.77), and this effect was also seen when restricting the analyses to the post-treatment period (), but not in the period beyond 15 years from treatment start (, Supplementary Table 3). When tumors diagnosed as lung adenocarcinoma and unspecified lung cancers were excluded from the analysis the incidence was still reduced among patients treated with five years of tamoxifen as compared with two years treatment (HR 0.36, 95% CI 0.18–0.73). Looking at specific histological types, the risk reduction appeared most pronounced for squamous cell and small cell lung cancer ().
Discussion
Results from randomized trials indicate that treatment with tamoxifen for primary breast cancer reduces the risk for contralateral breast cancer. However, less is known about the duration of the risk reduction [Citation1]. In our study, the risk-reducing effect on new cancer in contralateral breast was statistically significant also when restricting the analysis to the post-treatment period, and it seemed to be present up to 10 years after termination of active treatment. Further evidence for such a long-term effect of tamoxifen is given in the most recent update of the IBIS-I breast cancer prevention trial, comparing tamoxifen five years versus placebo and including women 35–70 years of age with increased risk for breast cancer, where the benefit of tamoxifen was fairly constant over time and remained significant even in the period after 10 years from start of treatment [Citation4].
Tamoxifen use increases the risk of endometrial cancer and the excess risk increases with longer use. The relative risk decreases after ending the treatment but it is still not clear how long an excess remains [Citation2]. In the present study, the risk of endometrial cancer was tripled during tamoxifen treatment. The relative risk then dropped and was no longer significantly different from unity, but the incidence tended to be increased in the five-year group even more than five years after treatment stopped. Similar tendencies have been seen both in a recent meta-analysis of adjuvant trials of five years versus no tamoxifen [Citation5] and in the IBIS-I prevention trial [Citation4].
In the present study, a statistically significant reduction of lung cancer incidence was found in the five-year group compared to the two-year group and the reduction was observed up to 10 years after termination of active treatment. In some cases, tumors diagnosed as lung adenocarcinoma or unspecified lung cancer may represent recurrences of breast cancer misclassified as lung cancer. When we excluded these subtypes from the analysis, the incidence was still reduced among patients treated with five years of tamoxifen as compared with two years treatment. A study of register-based data from Switzerland showed that women diagnosed with breast cancer treated with anti-estrogen, mainly tamoxifen, compared to a general population had a non-significant decrease in lung cancer incidence and a significant decrease in lung cancer mortality [Citation6]. We have not found any reports of tamoxifen reducing lung cancer risks in randomized trials. Instead, in the most recent update of the Stockholm adjuvant trial of two years of tamoxifen versus no tamoxifen, there was a significantly increased incidence of primary lung cancer in the tamoxifen group compared to controls (21 cases vs. four) [Citation7]. The authors have commented that this excess ‘typically concerned adenocarcinomas and tumors diagnosed during the first 10 years of follow-up’. This should be noted especially as the risk reduction observed in our study appeared more pronounced in squamous cell and small cell cancers. It suggests that future studies trying to further evaluate the effect of tamoxifen use on lung cancer risk should consider both histology and time of occurrence.
In summary, this long-term follow-up of a trial of postmenopausal patients with early stage breast cancer who were randomized to five years of adjuvant tamoxifen compared with two years of treatment showed a lower incidence of contralateral breast cancer and of lung cancer up to 10 years after treatment stopped. An excess risk for endometrial cancer was observed, but this decreased over time. The finding of a reduced risk of lung cancer is new, from a randomized trial setting, and further studies taking histologic type into account are needed.
IONC_1273547_supplementary_tables.zip
Download Zip (39.3 KB)Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Mellemkjaer L, Steding-Jessen M, Fredriksen K, et al. Risk of contralateral breast cancer after tamoxifen use among Danish women. Ann Epidemiol. 2014;24:843–848.
- Swerdlow AJ, Jones ME. Tamoxifen treatment for breast cancer and risk of endometrial cancer: a case-control study. J Natl Cancer Inst. 2005;97:375–384.
- Nordenskjöld B, Rosell J, Rutqvist LE, et al. Coronary heart disease mortality after 5 years of adjuvant tamoxifen therapy: results from a randomized trial. J Natl Cancer Inst. 2005;97:1609–1610.
- Cuzick J, Sestak I, Cawthorn S, et al. Tamoxifen for prevention of breast cancer: extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol. 2015;16:67–75.
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Davies C, Godwin J, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784.
- Bouchardy C, Benhamou S, Schaffar R, et al. Lung cancer mortality risk among breast cancer patients treated with anti-estrogens. Cancer. 2011;117:1288–1295.
- Rutqvist LE, Johansson H. Stockholm Breast Cancer Group. Long-term follow-up of the randomized Stockholm trial on adjuvant tamoxifen among postmenopausal patients with early stage breast cancer. Acta Oncol. 2007;46:133–145.
