Abstract
Background: Exercise during and after breast cancer treatment has shown several health benefits. However, little is known about the courses, patterns, and determinants of physical activity of breast cancer patients, and the role of exercise interventions on their physical activity behavior in the long run.
Material and methods: Self-reported physical activity was assessed in 227 breast cancer survivors before, during, and three, six, and 12 months post-intervention within two randomized resistance exercise trials performed during adjuvant chemo- or radiotherapy, respectively, with similar designs. Multiple ordinal logistic regression analyses were performed to identify determinants of physical activity at these time points.
Results: While the intervention group exercised a median 1.8 h/week during adjuvant therapy (interquartile range 1.4–2.5), 68% of controls did not engage in any exercise. At 12-months follow-up 32% of patients did not engage in any exercise irrespective of the intervention. Of the patients who cycled for transportation pre-diagnosis about half stopped cycling in the long term in both groups. In contrast, walking was maintained over time. Major determinants of low levels of exercise at 12-months follow-up were low pre-diagnosis levels of exercise, lower education, being postmenopausal, and having breast problems or depressive symptoms. Further, the intervention appeared to influence the type of sports performed, with strength exercise being the most common type of exercise at follow-up in the exercise group, more frequently compared to the control group.
Conclusion: The exercise intervention effectively countervailed the decrease in physical activity during cancer therapy and boosted strength exercise in the months following the intervention, but in the longer term many survivors were insufficiently active. Breast cancer survivors may need continued motivation and practical support tailored to their individual characteristics and physical activity history to incorporate exercise in everyday routine in the long term.
Exercise during and after breast cancer treatment probably results in less fatigue and improved physical fitness [Citation1,Citation2]. Observational studies and an exploratory follow-up of a randomized controlled trial (RCT) further suggest that physically active cancer survivors have better overall and disease-free survival and lower risk of cardiovascular disease, which is a major cause of death in breast cancer survivors [Citation3]. Thus, physical activity is highly recommended for breast cancer survivors. Nevertheless, in the past breast cancer survivors tended to rest and decrease their pre-diagnosis activity level especially during adjuvant therapy [Citation4]. Exercise interventions in the context of clinical trials typically increase (or prevent therapy-related decline of) the activity level of most participants in the course of the intervention. However, while physical activity behavior of cancer patients in general has been studied for over a decade [Citation5], data on the maintenance of physical activity in the long term beyond the duration of an intervention is still scarce and inconclusive [Citation6,Citation7]. While some studies reported a sustained effect [Citation8,Citation9], others observed insufficient activity levels or declined levels several months post-intervention [Citation10,Citation11].
A better understanding is needed regarding the change in physical activity behavior over the course of breast cancer diagnosis, treatment, rehabilitation, and survivorship. The identification of subgroups especially vulnerable to physical inactivity could help to develop more effective and better tailored strategies to improve the physical activity behavior in breast cancer survivors in the long run.
Therefore, we investigated the course, patterns, and determinants of exercise and of (non-sportive) walking and cycling for transportation at different times along the cancer continuum, assessed in two randomized exercise intervention trials for breast cancer patients during adjuvant chemotherapy (BEATE study) or radiotherapy (BEST study) with similar designs [Citation12,Citation13]. Participants randomized to the exercise intervention group had successfully adopted a 12-week supervised progressive resistance training (median attendance: 18 of 24 scheduled sessions). Both trials have shown significant benefits of the intervention on fatigue and some quality of life aspects in comparison to a relaxation group [Citation14,Citation15]. We now explored the follow-up data of these RCTs regarding the sustained impact of the exercise intervention and of individual factors on the physical activity behavior of breast cancer survivors up to 12 months post-intervention.
Material and methods
Study design and population
The analyses are based on two randomized controlled exercise intervention trials in breast cancer patients conducted in parallel between 2010 and 2013 at the National Center for Tumor Diseases (NCT) Heidelberg, Germany (BEATE study and BEST study). The conduct of the follow-up was a priori planned in the study protocol. Details of the study rationales, CONSORT flowcharts, and primary results have been published [Citation12–15]. For the present analyses data could be pooled as the interventions and the assessment of physical activity and investigated covariates were identical. In both trials patients had been randomized either to a resistance exercise (EX) or a relaxation control group (RC) in parallel to patients’ adjuvant therapy. The studies differed in that BEATE recruited breast cancer patients at the beginning of adjuvant chemotherapy in the metropolitan area around Heidelberg whereas BEST included patients scheduled for adjuvant radiotherapy at the NCT Heidelberg. Both studies were in accordance with the Helsinki Declaration, approved by the ethics committee of the University of Heidelberg, and registered at ClinicalTrials.gov (NCT01106820, NCT01468766). All participants gave written informed consent.
Interventions
The EX intervention comprised eight different machine-based progressive resistance exercises (three sets, 8–12 repetitions at 60–80% of one repetition maximum). The RC group performed progressive muscle relaxation according to the Jacobson method. Both interventions were performed for about one hour twice weekly over 12 weeks together with other cancer patients under the supervision and guidance of experienced therapists in specific training facilities in Heidelberg and the surrounding metropolitan area. After the primary endpoint assessments (Week 13) patients of the RC group also had the opportunity to perform the same supervised 12-week progressive resistance training. Patients in EX had the possibility to continue the resistance training upon a moderate membership fee.
Physical activity assessment
Frequency (days per week or days per month, and number of active months), duration (hours per day), and intensity (light, moderate, partly vigorous, mainly vigorous) of walking, cycling, and exercise including type of exercise were self-reported by standardized questions adopted from the SQUASH [Citation16] and modified for the specific treatment situations. Walking included walks of at least 20 minutes, for example for errands, going to work or for a stroll, or walking the dog. Nordic or sportive walking was reported as exercise. Likewise, mountain biking and racing cycling was counted as exercise, whereas the variable cycling included all other leisure or everyday cycling for transportation or pleasure. Activity was assessed pre-intervention, for instance after breast surgery but before start of radiotherapy (BEST study) or at begin of chemotherapy (BEATE study). Activity during the 12-week intervention period (excluding the resistance training of the intervention) was recorded in Week 13. The intervention adherence was documented throughout the training period. Activity post-intervention was assessed at three, six (only BEST), and 12-months follow-up. In addition, activity in the year prior diagnosis was assessed retrospectively at baseline.
Assessment of covariates
Sociodemographics, concomitant diseases, concomitant medication, smoking, exercise in adolescence, tumor characteristics, and cancer treatment were recorded by questionnaire or extracted from the medical charts. Education was classified as ‘advanced’ if women had an upper high school graduation or an academic degree, and classified as ‘basic’ otherwise. Body mass index (BMI) was calculated from measured height and weight as kg/m2. Fatigue was assessed by the self-administered 20-item Fatigue Assessment Questionnaire (FAQ) [Citation17]. Quality of life was assessed by the EORTC QLQ-C30 and -BR23 questionnaires including emotional aspects and typical symptoms during therapy such as pain or breast problems. Breast problems comprised pain, swelling, oversensitivity, and skin problems in the affected breast, and have been categorized as ‘no’ if the EORTC breast symptom score was below 25% (on a 0–100 scale) and otherwise as ‘yes’. The 20-item Center for Epidemiological Studies Depression Scale (CES-D) was used to measure depressive symptomatology with 16 points on a 0–60 scale as cutoff for mild depressive symptoms [Citation18]. Perceived social support was assessed by the multidimensional scale of perceived social support (MSPSS) [Citation19]. Cardiorespiratory fitness (VO2peak) was measured by spiroergometry [Citation20], and isometric and isokinetic muscle strength using an ISOMED2000®.
Statistical methods
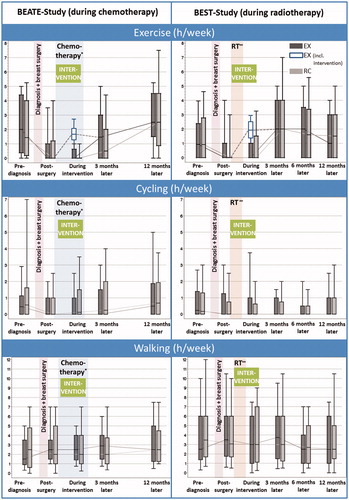
The average hours per week engaged in exercise, cycling, or walking were calculated as well as the average metabolic equivalent (MET)-h/week where the reported activities and intensities were weighted by the appropriate MET based on the compendium by Ainsworth. The distributions of exercise, cycling, and walking were presented separately as Box-Whisker plots with the boxes indicating the first and third quartiles, the middle line the median, and the Whisker ends the 10th and 90th percentile.
Further, due to skewness and zero-inflation exercise and cycling were categorized as none, >0–1.5 h/week, >1.5–3 h/week, and >3 h/week. Multiple ordinal logistic regressions were performed with categorized exercise or cycling at follow-up as dependent variables, respectively. All models included the intervention group (EX vs. RC) and study (BEATE vs. BEST) to adjust for potential differences in participant selection and treatment modalities. Based on possible or plausible associations the models included at least one factor of the following groups that were explored as potential determinants: age-related factors (age continuous, age categorized, menopausal status); sociodemographic factors (education, marital status, number of children <18 years, living alone or with others, social support); baseline fitness factors (BMI, weight, height, exercise in adolescence, VO2peak, muscle strength); psychological factors and quality of life at baseline (depressive symptoms, use of psychopharmaceuticals, fatigue, EORTC emotional function, pain, breast problems); cancer or treatment-related factors (tumor stage, receptor status, type of surgery, hormone therapy, herceptin therapy, taxane-containing therapy, stay at a rehabilitation clinic); preexisting diseases (cardiovascular diseases, chronic lung diseases, thyroid disorders), and smoking status (no smoking/quit last year/current smoking). In case of collinear variables only the stronger determinant from a group was kept in the final model. Cumulative odds ratios (OR) with 95% CIs were calculated. The proportional odds assumption was not violated. Potential effect modification by study or pre-diagnosis exercise was explored by including interaction terms in the model and by subgroup analyses.
All statistical tests were two-sided, and p < 0.05 was considered statistically significant. SAS Version 9.4 was used for all analyses.
Results
Of the 261 breast cancer patients randomized within the BEATE (n = 101) and BEST (n = 160) studies to EX (n = 132) or RC (n = 129), the physical activity questionnaire was completed by 99% (EX: n = 131, RC: n = 128) at baseline, by 96% for the intervention period (EX: n = 127, RC: n = 124, seven dropouts, one death), by 91% at three-months follow-up (EX: n = 120, RC: n = 117, six additional dropouts, eight did not complete the questionnaire), by 93% at six-months follow-up (only BEST study, EX: n = 75, RC: n = 73, four additional dropouts), and 227 (87%) completed both the pre-diagnosis and the 12-month post-intervention physical activity questionnaire (EX: n = 114, RC: n = 113). Those n = 227 patients were included in the analyses.
Patients’ and treatment characteristics were similarly distributed in EX and RC (). At baseline participants were on average 55 ± 9 years old, mainly non-smokers, 20.7% were obese (BMI ≥30 kg/m2), 44.1% had completed at least upper high school, and only 11.5% were living alone. Depressive symptoms were observed in 41.9% of patients.
Table 1. Baseline and treatment characteristics of the follow-up population and patients lost-to-follow-up.
The 34 patients lost to the 12-month follow-up significantly differed from the follow-up population in having higher tumor stages (p = 0.0015), being more likely to smoke currently or recently (p = 0.025), and including a larger proportion who had not done any exercise pre-diagnosis (p = 0.038) ().
Exercise intervention attendance
In EX patients attended a median of 18 (interquartile range 13–23) of the 24 scheduled sessions concomitant to the adjuvant therapy. About 40% of patients in the EX intervention group continued the supervised resistance exercise after the end of the intervention period at their own cost at least for a few weeks, some even for several months or permanently. In the RC waitlist group, 25% of patients reported resistance exercise in the follow-up, for instance after the primary endpoint assessments in Week 13. The majority of them conducted the same training as EX in the Heidelberg training center at least for some weeks, the others at local facilities. The RC participants engaging in resistance training differed from those not using the waitlist offer for a resistance training by being better educated (75% vs. 35% advanced education) and having a lower BMI (61% vs. 39%, BMI <25).
Satisfaction with interventions
Both interventions (EX, RC) were rated by the majority of participants as positive or very positive with respect to support by the trainer (83%, 81%), training location (72%, 70%), duration of training (78%, 80%), frequency of training (80%, 74%), and structure of the training (77%, 80%). Likewise, psycho-social aspects associated with the interventions were rated as (very) positive by most participants regarding contact to other patients undergoing therapy (55%, 68%), structuring of the week by a training twice per week (73%, 66%), and becoming pro-active themselves during cancer therapy (89%, 83%). Among the EX group receiving feedback on the own physical fitness was perceived as (very) positive by 83% of participants versus 55% in RC. Percentages are based on all distributed questionnaires (including missing answers).
Exercise, cycling, and walking over time
As presented in , the time engaged in exercise before the cancer diagnosis (median 1.3 h/week) decreased substantially after diagnosis and surgery (median 0 h/week). In RC the majority of patients (68.1%) did not engage in any exercise especially during chemotherapy (77.3%). In contrast, patients in the EX group engaged in exercise a median time of 1.8 h/week (interquartile range 1.4–2.5) during adjuvant therapy, mainly within the interventional resistance training. Some months after adjuvant therapy the time engaged in any exercise was similar or even slightly higher than before diagnosis irrespective of the intervention group assignment. Time spent with cycling for transportation was already low before diagnosis (median 0.4 h/week), decreased further after diagnosis and surgery (median 0.0 h/week) and recovered only slightly over time. In contrast, patients’ walking time remained rather stable over the entire time frame with medians around 2.5–3.0 h/week. Overall, there were no substantial differences between both intervention groups. Results considering MET-hours per week instead of hours per week were similar.
Figure 1. Box-Whisker plots of hours per week spent with exercise and cycling or walking for transportation at different time periods. Dark gray: exercise intervention group (EX), including only activities beyond the study intervention. White: EX including in addition the resistance exercise within the study intervention. Light gray: relaxation control group (RC). *Chemotherapy followed by a subsequent radiotherapy in 84% of BEATE participants. **Radiotherapy (RT) in BEST was in part after a previous adjuvant (9%) or neoadjuvant (13%) chemotherapy.
Temporal or long-term cessation of exercise and cycling
Proportions of women engaging in any exercise (apart from the resistance exercise of the intervention) or any cycling were almost identical in EX and RC. About two-thirds of the patients engaged in exercise within the year before diagnosis in both groups (). More than half of them stopped these exercise activities after diagnosis and breast surgery. In the EX group 32% did not engage in any exercise 12 months after the intervention, including women who had been active pre-diagnosis and who had a good intervention adherence. Among RC participants the proportion was similar (28%). However, about half of the women without any exercise in the year before diagnosis exercised at 12-months follow-up.
Figure 2. Proportion of patients engaging in any exercise or cycling, respectively, indicating changes from pre-diagnosis to the different periods post-diagnosis. For example, dark green bars indicate the proportion of patients who exercised pre-diagnosis but did not exercise in the considered time period; light green bars indicate the proportion of patients who exercised at both, pre-diagnosis and the considered time period.
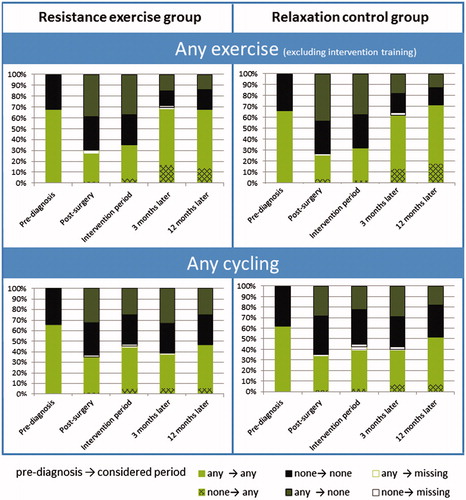
Cycling behavior was also similar in EX and RC. Overall, of the women who reported cycling for transportation in the year before diagnosis (64%) about half stopped cycling after diagnosis and/or surgery. Many of them did not readopt their previous cycling behavior, thus overall resulting in only 49% of breast cancer survivors using the bike for transportation at 12-months follow-up. Of those survivors who used the bike, the majority (86%) also engaged in any exercise, while among the non-bikers only 54% performed any exercise.
Types of exercise
In the year before the cancer diagnosis most reported sport activities were aerobic and callisthenic exercise and (Nordic) walking/jogging. At 12-months follow-up the most frequently reported exercise in RC still were similar as pre-diagnosis. In contrast, in EX 12-months post-intervention those types of exercise were topped by resistance exercise, and exercise was mostly performed in a sports club or fitness center whereas pre-diagnosis it was mainly performed privately with friends or alone. Ball or team sports were generally rare before as well as after diagnosis and intervention.
Determinants of physical inactivity
Multiple ordinal logistic regressions () revealed as strongest determinant of exercise 12 months post-intervention the pre-diagnosis level of exercise with OR (none vs. >1.5–3 h/week) = 0.25 (95% CI 0.12, 0.54, p < 0.001). Further, being postmenopausal [OR 0.38 (0.16, 0.90)], lower education [OR 0.44 (0.26, 0.76)], and having breast problems at baseline [OR 0.48 (0.27, 0.84)] showed significantly decreased odds for exercise at follow-up. Depressive symptoms at baseline also tended towards lower odds for exercise [OR 0.59 (0.34, 1.02)] and reached statistical significance when excluding the correlated variable breast problems from the model. Age (categorized or continuous) showed no significant association, even without controlling for menopausal status. Fatigue, pain, muscle strength, and VO2peak were also no significant determinants of exercise in the follow-up period, neither when considering the levels at end of intervention nor the changes from baseline to end of intervention. Participants from the BEATE study exercised significantly more than participants from BEST, possibly reflecting a selection of more active and motivated patients by different mode of recruitment. Yet, there was no significant effect modification by study (BEATE vs. BEST) for any of the above mentioned determinants.
Table 2. Multiple ordinal logistic regressions on exercise level 12 months post-interventionTable Footnotea.
Significant interaction was only seen between pre-diagnosis sport levels and BMI (p = 0.0010). In the subgroup of participants who had exercised >1.5 h/week prior to their cancer diagnosis a higher BMI was a significant determinant for little exercise at follow-up with OR (≥30 vs. <25 kg/m2) = 0.28 (0.082, 0.94). In contrast, in the subgroup of women who were inactive before diagnosis BMI showed no association with exercise in the follow-up. Categorization of exercise activities by MET-hours per week instead of hours per week yielded similar results.
Exploring the impact of the intervention satisfaction ratings indicated only a borderline significant association between rating a structuring of the week by the training as (very) positive and higher exercise level at follow-up (p = 0.078).
Ordinal logistic regressions considering cycling categories showed also pre-diagnosis cycling as significant determinant of cycling at 12-months follow-up with OR (none vs. >1.5–3 h/week) = 0.05 (0.02, 0.14, p < 0.0001). The only other borderline significant determinant was education with OR (basic vs. advanced) = 0.58 (0.32, 1.05). Logistic regression on cycling cessation in the subgroup of patients who cycled before diagnosis did not reveal any clear determinants (data not shown).
Discussion
This follow-up of two randomized controlled exercise intervention studies including 227 breast cancer patients investigated the course, patterns, and determinants of physical activity at different time points along the cancer and survivorship continuum. After diagnosis and breast surgery exercise levels markedly decreased, and without an exercise intervention (i.e. in the control group) levels remained low during adjuvant therapy. Yet about one year later time spent with exercise was back to pre-diagnosis levels. Similar patterns have been observed in earlier studies [Citation4,Citation21]. In contrast, patients in the intervention group exercised a median 1.8 h/week even during adjuvant therapy. However, the times spent with exercise 12 months post-intervention did not differ from the control group. It should be noted that almost one-third of breast cancer survivors did not engage in any exercise about one year after adjuvant therapy, even when having received the supervised progressive resistance training concomitant to therapy. This is in line with another randomized exercise intervention trial in breast cancer patients during adjuvant therapy that observed that 42% of participants did not meet exercise guidelines six months post-intervention, irrespective of intervention group [Citation11]. Mutrie et al. also observed no significant difference in leisure time physical activity six and 18 months post-intervention between breast cancer survivors randomized to a 12-week exercise group during adjuvant therapy or usual care control group [Citation22]. A reason could be that the therapy phase reflects exceptional circumstances, for example being on sick leave, having more time, and focusing more on healthy behavior, but after recovery most breast cancer patients resume their previous everyday tasks in their families and at work. Thus, in the long run time spent with exercise may often be similar to pre-diagnostic levels. In contrast to exercise interventions during adjuvant therapy, interventions several months or years after the end of cancer treatment have shown significantly increased physical activity levels also several months post-intervention compared to baseline. However, those interventions typically included sedentary breast cancer survivors likely intending to become more active [Citation6,Citation8,Citation9]. Getting people physically active in the long term appears to be difficult not only in breast cancer survivors. Also, among healthy postmenopausal women only 62% were active 12 months after a year-long structured exercise intervention (ALPHA trial) comparable to the control group (58%) [Citation23]. Overall, an implication from our study for practice is that the cancer diagnosis might be used as a ‘teachable moment’ to motivate breast cancer patients to engage in progressive resistance training, yet, an exercise intervention alone is not sufficient for long-term maintenance of an appropriate physical activity level. It appears crucial for exercise interventions to incorporate steps aiming to integrate exercising in everyday routine after completion of cancer therapy. For example, for some patients it could be helpful to become permanently integrated in a fitness group where they feel comfortable doing exercises, have good social contacts, and regular training schedules. Moreover, theory-based behavioral techniques and contact to positive role models might be a promising approach [Citation24].
Regarding cycling, which is a common means of transportation in Germany, also a marked decline was observed after diagnosis. Before diagnosis, 64% of the participants had used a bike for transportation. About half of them stopped cycling after diagnosis or breast surgery and many did not take it up again in the long term, irrespective of the intervention, overall resulting in only 47% using the bike 12 months post-intervention. Our results are similar to an observational study of 1067 postmenopausal breast cancer survivors in Germany showing 57% using the bike pre-diagnosis, only 19% during adjuvant treatment, and 50% one year after surgery [Citation4]. An implication of those results is that steps needs to be taken that after breast cancer treatment women (again) use the bike for everyday cycling for transportation or pleasure. In our study neither age, BMI, nor any sociodemographic, physical, psychological, quality of life, or medical factors showed a significant association with cycling duration or cycling cessation. Thus, the reasons for cycling cessation remain unclear and may be manifold, for example pain, fatigue, concerns about lymphedema, or potentially feeling no longer confident to ride the bike in traffic due to dizziness, polyneuropathy, or weakness, or just getting used during therapy to take the car instead of the bike for transportation. Addressing these issues, encouragement, and offering practical support, such as easy bicycle tours for cancer survivors, might help to reduce long-term cessation of cycling after breast cancer therapy.
In contrast to exercise and cycling, the time spent with non-sportive walking, for example for errands, to go to the doctor, to go for a stroll or walk the dog, remained stable throughout the considered period. As in Germany, there are commonly sidewalks and trails in all regions, going for a walk is a convenient form of everyday physical activity even during adjuvant therapy.
Although there were no significant group differences with respect to time and intensity of exercise in the follow-up period, the resistance training intervention appeared to affect the type of exercise. After the intervention breast cancer survivors conducted more strength exercise, which was the most common type of exercise at follow-up in the exercise intervention group and more frequent compared to the control group. The resistance training intervention was appraised by the majority of participants as (very) positive with regard to its duration, frequency, design, and psycho-social aspects. It had shown significant benefits with respect to muscle strength, fatigue, and several quality of life domains, and the adherence and the feedback to the intervention was good [Citation14,Citation15]. Maintaining muscle strength is important for overall health and quality of life [Citation25], but in the past exercise performed by cancer survivors often included mainly aerobic tasks such as walking, jogging, or swimming. Engaging into structured resistance exercise might even provide survival benefits beyond maintaining an overall physically active lifestyle in cancer survivors [Citation26]. Therefore, interventions focusing also on resistance training seem advisable.
Data on determinants of exercise maintenance are still scarce and inconclusive [Citation6]. We identified low pre-diagnosis levels of exercise, lower education, being postmenopausal, and having breast problems and depressive symptoms at baseline as major determinants of low level of exercise at 12-months follow-up. Pre-diagnosis exercise was also a major determinant of later physical activity in other trials [Citation11,Citation27]. Education had shown a positive association with exercise maintenance in a previous study, whereas in two other studies no association was observed [Citation6]. Associations with menopausal status have not been investigated previously, but age was shown to be inversely associated with exercise maintenance in two of three studies [Citation6]. Data on breast problems as potential determinant have not been presented previously. Depressive symptoms were a stronger predictor of exercise than the correlated symptom fatigue in our study, whereas another study observed fatigue as a stronger determinant than depression [Citation11]. Overall, another implication for practice is that especially breast cancer survivors who are less educated, postmenopausal, or did not exercise before diagnosis as well as those who suffer from breast problems or depressive symptoms need increased attention and support to maintain exercise after cancer therapy and intervention cessation. For these survivor groups one might consider a combination of a (resistance) exercise intervention with a behavioral intervention to promote structured exercise and overall increased physical activity.
The BEATE and BEST studies have previously shown that after a chemotherapy patients’ cardiorespiratory fitness and muscle strength is substantially reduced [Citation20,Citation28], and that a resistance training concomitant to adjuvant therapy can improve muscle strength or ameliorate its therapy-associated decline [Citation29], has beneficial effects on patient-reported outcomes such as fatigue and pain [Citation14,Citation15], and to some extent on inflammatory processes [Citation30]. Therefore, our intervention focusing on well performed progressive resistance exercise training appears important and effective with respect to physiological and psychological factors, but it should be improved with respect to long-lasting physical activity behavior to maintain health benefits in the long run.
As main limitation of our study it should be noted that the analysis of the exercise intervention maintenance is limited insofar as the patients in the waitlist control group were offered the resistance training program after completion of the initial 12-week period. This could have led to an underestimation of the exercise intervention effect on physical activity behavior at follow-up. However, only about 25% of controls started with the resistance training. Further, the physical activity questionnaire had not been validated in the used form. However, as described above, it was based on existing validated questionnaires and modified to match our patient settings. Exercise was recorded as free text, enabling exploration of which types of exercise have been performed, yet the descriptions provided by the participants were not always precise. Moreover, the study population may differ from the overall breast cancer survivor population by being of younger age, more educated, more motivated and generally willing to exercise during therapy. Thus, levels of physical activity might be even lower in the overall survivor population than observed in our study. Although the sample size was relatively large compared to other follow-up studies of intervention trials, only cumulative ORs of 2.0 or above (or vice versa 0.50 or below) might have been detectable with sufficient power. Thus, factors with a lower impact on physical activity behavior may have been missed. Strengths of our study include a high response rate at 12-months follow-up of 87%, comprehensive assessment of physical activity at several time points including besides exercise also walking and cycling for transportation, and a comprehensive assessment of co-factors enabling exploration of determinants.
In conclusion, breast cancer patients reduced their engagement in exercise and cycling for transportation after diagnosis and treatment. Yet, the resistance training intervention effectively countervailed this decrease in physical activity during cancer therapy and boosted strength exercise in the months following the intervention. However, in the long run there was no significant group difference in the time spent exercising and still many survivors were insufficiently active. Thus, breast cancer survivors, especially those who engaged in little or no exercise before diagnosis, are less educated, postmenopausal, or are more likely having breast problems or depressive symptoms, may need continued motivation and practical support tailored to their individual characteristics and physical activity history to incorporate exercise in everyday routine in the long term.
Acknowledgments
The authors thank the study participants who willingly spent their time to complete the study procedures; Dr Karin Potthoff, Dr Oliver Klassen, Petra Armbrust, Dr Sabine Wessels, Dr Jan Oelmann, Werner Diehl, Lena Kempf, Marcel Bannasch, Nadine Ungar, Beate Biazeck, and Renate Schoenmakers for the successful realization of the studies; the participating breast centers for supporting the recruitment and the Institute of Sports and Sports Science of the University of Heidelberg for providing the training facilities.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Jones LW, Alfano CM. Exercise-oncology research: past, present, and future. Acta Oncol. 2013;52:195–215.
- Furmaniak AC, Menig M, Markes MH. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst Rev. 2016;9:CD005001.
- Lahart IM, Metsios GS, Nevill AM, et al. Physical activity, risk of death and recurrence in breast cancer survivors: a systematic review and meta-analysis of epidemiological studies. Acta Oncol. 2015;54:635–654.
- Bock C, Schmidt ME, Vrieling A, et al. Walking, bicycling, and sports in postmenopausal breast cancer survivors-results from a German patient cohort study. Psychooncology. 2013;22:1291–1298.
- Irwin ML, Crumley D, McTiernan A, et al. Physical activity levels before and after a diagnosis of breast carcinoma: the Health, Eating, Activity, and Lifestyle (HEAL) study. Cancer. 2003;97:1746–1757.
- Kampshoff CS, Jansen F, van Mechelen W, et al. Determinants of exercise adherence and maintenance among cancer survivors: a systematic review. Int J Behav Nutr Phys Act. 2014;11:80.
- Jankowski CM, Ory MG, Friedman DB, et al. Searching for maintenance in exercise interventions for cancer survivors. J Cancer Surviv. 2014;8:697–706.
- May AM, Korstjens I, van Weert E, et al. Long-term effects on cancer survivors' quality of life of physical training versus physical training combined with cognitive-behavioral therapy: results from a randomized trial. Support Care Cancer. 2009;17:653–663.
- Demark-Wahnefried W, Morey MC, Sloane R, et al. Reach out to enhance wellness home-based diet-exercise intervention promotes reproducible and sustainable long-term improvements in health behaviors, body weight, and physical functioning in older, overweight/obese cancer survivors. J Clin Oncol. 2012;30:2354–2361.
- Ottenbacher AJ, Day RS, Taylor WC, et al. Long-term physical activity outcomes of home-based lifestyle interventions among breast and prostate cancer survivors. Support Care Cancer. 2012;20:2483–2489.
- Courneya KS, Friedenreich CM, Reid RD, et al. Predictors of follow-up exercise behavior 6 months after a randomized trial of exercise training during breast cancer chemotherapy. Breast Cancer Res Treat. 2009;114:179–187.
- Schmidt ME, Wiskemann J, Krakowski-Roosen H, et al. Progressive resistance versus relaxation training for breast cancer patients during adjuvant chemotherapy: design and rationale of a randomized controlled trial (BEATE study). Contemp Clin Trials. 2013;34:117–125.
- Potthoff K, Schmidt ME, Wiskemann J, et al. Randomized controlled trial to evaluate the effects of progressive resistance training compared to progressive muscle relaxation in breast cancer patients undergoing adjuvant radiotherapy: the BEST study. BMC Cancer. 2013;13:162.
- Schmidt ME, Wiskemann J, Armbrust P, et al. Effects of resistance exercise on fatigue and quality of life in breast cancer patients undergoing adjuvant chemotherapy: a randomized controlled trial. Int J Cancer. 2015;137:471–480.
- Steindorf K, Schmidt ME, Klassen O, et al. Randomized controlled trial of resistance training in breast cancer patients receiving adjuvant radiotherapy: results on cancer-related fatigue and quality of life. Ann Oncol. 2014;25:2237–2243.
- Wendel-Vos GC, Schuit AJ, Saris WH, et al. Reproducibility and relative validity of the short questionnaire to assess health-enhancing physical activity. J Clin Epidemiol. 2003;56:1163–1169.
- Beutel ME, Hinz A, Albani C, et al. Fatigue assessment questionnaire: standardization of a cancer-specific instrument based on the general population. Oncology. 2006;70:351–357.
- Weissman MM, Sholomskas D, Pottenger M, et al. Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol. 1977;106:203–214.
- Dahlem NW, Zimet GD, Walker RR. The multidimensional scale of perceived social support: a confirmation study. J Clin Psychol. 1991;47:756–761.
- Klassen O, Schmidt ME, Scharhag-Rosenberger F, et al. Cardiorespiratory fitness in breast cancer patients undergoing adjuvant therapy. Acta Oncol 2014;53:1356–1365.
- Littman AJ, Tang MT, Rossing MA. Longitudinal study of recreational physical activity in breast cancer survivors. J Cancer Surviv. 2010;4:119–127.
- Mutrie N, Campbell A, Barry S, et al. Five-year follow-up of participants in a randomised controlled trial showing benefits from exercise for breast cancer survivors during adjuvant treatment. Are there lasting effects? J Cancer Surviv. 2012;6:420–430.
- Aparicio-Ting FE, Farris M, Courneya KS, et al. Predictors of physical activity at 12 month follow-up after a supervised exercise intervention in postmenopausal women. Int J Behav Nutr Phys Act. 2015;12:55.
- Stacey FG, James EL, Chapman K, et al. A systematic review and meta-analysis of social cognitive theory-based physical activity and/or nutrition behavior change interventions for cancer survivors. J Cancer Surviv. 2015;9:305–338.
- Strasser B, Steindorf K, Wiskemann J, et al. Impact of resistance training in cancer survivors: a meta-analysis. Med Sci Sports Exerc. 2013;45:2080–2090.
- Hardee JP, Porter RR, Sui X, et al. The effect of resistance exercise on all-cause mortality in cancer survivors. Mayo Clin Proc. 2014;89:1108–1115.
- Vallance J, Plotnikoff RC, Karvinen KH, et al. Understanding physical activity maintenance in breast cancer survivors. Am J Health Behav. 2010;34:225–236.
- Klassen O, Schmidt ME, Ulrich CM, Schneeweiss A, Potthoff K, Steindorf K, Wiskemann J. Muscle strength in breast cancer patients receiving different treatment regimes. J Cachexia Sarcopenia Muscle. 2016. [cited 2016 Nov 28]. doi: 10.1002/jcsm.12165
- Wiskemann J, Schmidt ME, Klassen O, Debus J, Ulrich CM, Potthoff K, Steindorf K. Effects of 12-week resistance training during radiotherapy in breast cancer patients. Scand J Med Sci Sports. 2016. [cited 2016 Oct 5]. doi: 10.1111/sms.12777
- Schmidt ME, Meynkohn A, Habermann N, et al. Resistance exercise and inflammation in breast cancer patients undergoing adjuvant radiation therapy: mediation analysis from a randomized, controlled intervention trial. Int J Radiat Oncol Biol Phys. 2016; 94:329–337.
