Abstract
Background: Axillary lymph node dissection (ALND) and adjuvant radiotherapy (RT) in early breast cancer are associated with a risk of morbidity, including lymphedema and impaired shoulder mobility. The aim of this study was to evaluate loco-regional morbidity after breast conserving surgery (BCS), ALND, taxane-based chemotherapy and whole breast irradiation (WBI) with or without regional nodes RT.
Material and methods: Eligible patients had BCS and ALND from 2007 to 2012 followed by adjuvant taxane-based chemotherapy and if indicated, trastuzumab and endocrine treatment. The RT consisted of WBI and regional nodes RT in case of ≥ pN1 disease (group 1) and WBI only in case of pN0-1(mic) disease (group 2). The dose was 50 Gy in 25 fractions. The patients were invited to participate in a cross-sectional study evaluating morbidity.
Results: Of the 347 eligible patients, 277 patients (79%) accepted the invitation. Of these, 185 patients (67%) belonged to group 1 and 92 patients (33%) to group 2. The median time from RT to evaluation of morbidity was 3.3 years (group 1) and 4.3 years (group 2). In group 1, 34 patients (18%) and in group 2, 15 patients (16%) had ≥2 cm enlargement in circumference of ipsilateral upper or lower arm (p = .67). The frequence of impairment of ipsilateral shoulder abduction to ≤120° was 3% in both groups and of shoulder flexion to ≤120° was 1% and 2% (group 1 versus 2). No difference in patient reported outcome measure (PROM) data regarding heaviness or enlargement of ipsilateral upper and lower arm or mobility and sensory disturbances.
Conclusion: The risk of lymphedema was low in patients after ALND and not related to use of regional nodes RT. Impairment of shoulder function was rare, and no differences in PROM were detected regarding use or not of regional nodes RT.
Introduction
The number of breast cancer survivors has increased especially due to early diagnosis and optimized multimodality treatment programs. Therefore, more focus is now on the late morbidity that inevitably follows treatment.
Lymphedema is a prominent complication of the loco-regional treatment of breast cancer, and may cause chronic physical morbidity following the diagnosis. Reporting of lymphedema is inconsistent in the literature due to lack of harmony on definition. Most studies are based on objective measurements and not on patient reported outcome measures (PROM), although one-fourth of studies in the recent systematic review report PROM [Citation1]. A PROM questionnaire for early breast cancer patients has been developed and used in a Danish nationwide cross-sectional study of 3754 women aged 18–70 years who received surgery from 2005 to 2006 and adjuvant therapy for early breast cancer [Citation2]. Swelling/heaviness of the ipsilateral arm was reported in 13–65% of the patients and was dependent on treatment group and associated with young age, axillary lymph node dissection (ALND) and radiotherapy (RT).
In general, the association between breast cancer treatment factors and lymphedema is complex and increased risk is described especially after mastectomy, ALND and RT [Citation3]. The risk of lymphedema has been reported the same among the following treatment modalities: axillary RT alone, axillary sampling plus RT and ALND, although the risk increases by far with the combination of ALND and regional nodes RT [Citation4].
In Denmark, there is consensus performing ALND in patients having clinically node-positive disease, and also in clinically node-negative disease with ≥1 macrometastasis in sentinel node (SN), or ≥3 SN with micrometastases, although the indication for ALND in selected patients is under dispute and now being investigated in the SENOMAC trial (www.senomac.se). RT is recommended in case of ≥1 macrometastasis, and the target volume includes CTVn_(I), II, III, IV, the internal mammary nodes (IMN) and the interpectoral nodes. The Danish Breast Cancer Group (DBCG) has performed a number of studies regarding morbidity after adjuvant breast cancer RT [Citation5–9]. In a morbidity study after hypofractionation (2 fractions per week compared with 5 fractions per week) using the NSD formula in 1977–1982 (nominal standard dose, which means that the total dose is adjusted based on the number of fractions) hypofractionation caused increased morbidity including impairment of shoulder movements and arm lymphedema [Citation5]. Since then, the DCBG has recommended normofractionation. However, from 2009 to 2014 the DBCG HYPO trial accrued 1883 patients treated with whole breast irradiation (WBI) (no axillary RT) being randomized to 50 Gy/25 fractions versus 40 Gy/15 fractions, and at 3 years follow up there was no excess morbidity using hypofractionation [Citation10]. Likewise, in the Canadian and British fractionation studies no differences in late morbidity were seen [Citation11–14]. In March 2015, the Skagen Trial 1 was initiated with the aim to reintroduce moderately hypofractionated loco-regional RT in Denmark in a quality-controlled and systematic manner. In the trial, patients treated with regional nodes RT are randomized to 50 Gy/25 fractions versus 40 Gy/15 fractions with the primary endpoint being 3-year risk of arm lymphedema. The study is ongoing as a multinational clinically controlled randomized trial. In order to estimate the number of patients needed to accrue in the Skagen Trial 1, knowledge on loco-regional morbidity in modern treated patients was needed. Therefore, this cross-sectional study was performed to estimate the magnitude of morbidity after modern breast cancer treatment and to decide relevant endpoints for the Skagen Trial 1.
The aim of this study was to evaluate the morbidity using (1) objective measurement and (2) PROM in two groups of modern treated adjuvant breast cancer patients, differing only regarding regional nodes RT or not and thereby being able to estimate the contribution of the morbidity by the regional nodes RT.
Material and methods
Every early breast cancer patient receiving adjuvant RT from 2007 to 2012 at Department of Oncology, Aarhus University Hospital and without recurrence were in 2014 invited by mail to participate in a cross-sectional study evaluating their morbidity based on both (1) objective and (2) PROM. This group consisted of 347 consecutive patients, who were selected based on the following criteria: breast conserving surgery (BCS), ALND, adjuvant chemotherapy including docetaxel (which became DBCG standard in 2007) and RT with or without regional nodes RT. The patients had trastuzumab and endocrine treatment if indicated based on the tumor receptor status. Regardless of T and N stage or preexisting morbidity (no routine reporting of morbidity was done in the standard Danish follow-up breast program), patients were included in the study. The study comprised: (1) a physical examination performed by a trained nurse (evaluating breast induration, bilateral measurements of arm circumference 15 cm above and 10 cm below the olecranon and bilateral arm mobility regarding abduction and flexion), and (2) PROMs were collected using a validated questionnaire, a Body Image Scale (BIS), and a DBCG questionnaire focusing especially on chronic pain, sensory disturbances, lymphedema, functional impairments and body image [Citation2,Citation15,Citation16].
Since the patients were selected based on their surgical procedure and the chemotherapy regimen (EC-TAX), the treatment schedules for these patients were as follows: The surgery consisted of BCS and ALND of level I and II. The indication for ALND was one or more micro- or macrometastases to sentinel node (SN) or the patients were clinically node positive. The adjuvant chemotherapy was initiated after surgery and consisted of three cycles of EC (epirubicin 90 mg/m2, cyclophosphamide 600 mg/m2) followed by three cycles of TAX (docetaxel 100 mg/m2) according to the DBCG 07 guidelines. In HER-2 positive patients, adjuvant intravenous trastuzumab was given every 3 weeks for one year. The endocrine treatment to ER positive patients began 3 weeks after chemotherapy and consisted of tamoxifen to premenopausal and letrozol to postmenopausal women.
RT was initiated 2–3 weeks after chemotherapy. In patients without macrometastases, the target area was the breast only (). The dose in all patients was either 48 Gy in 24 fractions (before 2009) or 50 Gy in 25 fractions. A boost of 16 Gy in 8 fractions was delivered to young patient’s ≤40 years or in case of a resection margin <2 mm. A boost of 10 Gy in 5 fractions was delivered to patients aged 41–49 years. In patients with ≥1 macrometastasis, the target areas were the breast and the regional lymph nodes including axilla level II, III and IV (). Level I was included in case of ≥6 macrometastases or <10 removed axillary nodes (). The IMN were included in right sided breast cancer patients and not in patients with left sided breast cancer in order to protect the heart [Citation17]. The technique used was tangential photon beams to the breast and IMN in IC 1-4 if indicated and an anterior photon beam to the axilla level I–IV. All plans were 3D planned, but comprehensive routine contouring of lymph nodes began in 2015, so no patients in this study had delineation of target volumes except for CTVn_L2 and CTVn_IMN. The cranial border regarding inclusion of level 4 lymph nodes were at that time the cricoid cartilage, the lateral border was the acromion and the medial border respected the larynx. The caudal border was matched to the tangential fields using half beam technique. The humeral head was protected by wedges from the field edge.
All patients were treated in supine position.
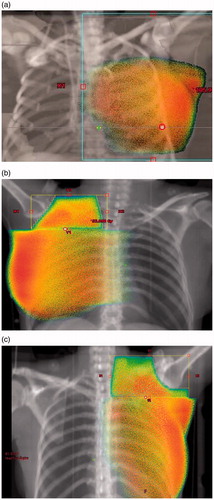
Figure 1. (a) WBI: 50% isodose. (b) Loco-regional RT: axillary level 1 not included; 50% isodose. (c) Loco-regional RT: axillary level 1 included; 50% isodose.
Lymphedema
The patients were asked about swelling or heaviness of the ipsi- and contralateral arm and/or hand. The objective measurements followed the standard DBCG screening procedure measuring the circumference 15 cm proximal and 10 cm distal to olecranon. In patients wearing an arm sleeve at the time of physical examination, the arm circumference was not measured routinely from the beginning of the study, but after further considerations and recognizing of that potential bias, measurements in 37 consecutive patients using arm sleeves (wearing no arm sleeve at least for 24 hours before measurements) were performed.
Arm mobility
The patients were asked to answer questions regarding their functionally capacity, both regarding daily routines as for example if they were able to wash their hair, put on a bra and continue previous sports activity. The physical examination performed by the nurse included measurement of arm mobility both flexion and abduction while the patient was placed in a standard position in front of a grading scale.
Statistics
The two groups were compared using chi-square statistics.
Results
Of the 347 invited patients, 277 accepted to participate in the study (79%). Group 1 comprised 185 patients (67%) treated with WBI and regional nodes RT, and group 2 comprised 92 patients (33%) treated with WBI. The median follow-up time from RT to morbidity registration was 3.3 years in group 1 and 4.3 years in group 2 since patients in group 2 were examined latest. The patient characteristics are shown in .
Table 1. Patient and tumor characteristics at diagnosis.
Lymphedema
In group 1, 34 patients (18%) and in group 2, 15 patients (16%) had ≥2 cm enlargement in circumference of ipsilateral upper or lower arm (p = .67). By measuring the contralateral arm 14 patients (8%) in group 1 and 3 patients (3%) in group 2 had ≥2 cm enlargement. There was no difference in use of arm sleeve between the groups (). Patient reported heaviness or enlargement of ipsilateral upper and lower arm was found in 51% and 47% (group 1 and 2) (p = .52).
Table 2. Objective and patient reported outcome measures on morbidity after loco-regional and systemic therapy of early breast cancer.
Impairments of shoulder movements
The frequence of impairment of ipsilateral shoulder function with abduction to ≤120° was 3% in both groups, and of shoulder flexion to ≤120° was 1% and 2% (group 1 and 2) (). A high percentage in both groups reported impairment in physical abilities (), however, no differences in PROM’s were seen between the groups.
In general, 59% and 49% in group 1 and 2 reported pain in the loco-regional area including the breast area, side of the chest wall, axilla or arm (). Most often the pain was located in the breast area, but one-third of the patients also reported pain on the side of the chest wall, in the axilla or arm. The median pain score on a VAS scale was 3 regarding pain in the breast area in both groups ().
Sensory disturbances
Regarding sensory disturbances only 21% of patients in both groups were without any symptoms (). The most common location of sensory disturbance or physical discomfort was the axilla and the ipsilateral arm. Also, a high number of patients reported pain or sensory disturbances in the feet or toes: 39% and 44% in group 1 and 2, respectively.
PROM’s collected from the DBCG questionnaire and BIS demonstrated no difference in body consciousness, dressing habits, self confidence, self image, physical appearance, femininity, difficulties in being naked, feeling less sexually attractive, preference of avoiding other people, the feeling of having a defect body, satisfaction with the body, the breast scar or the residual breast (data not shown).
Discussion
This cross-sectional study was performed in order to explore arm morbidity caused by the ALND or by the regional nodes RT to estimate morbidity after modern breast cancer treatment. In patients treated with both ALND and regional nodes RT, the 3-year risk of ipsilateral lymphedema was 18% in contrast to 16% in patients treated with ALND alone. Interestingly, data also showed that the risk of contralateral ‘lymphedema’ was 8% in patients treated with ALND and regional nodes RT compared with 3% in patients treated with ALND only. This indicates that women in general may have objective measurable differences between their arms not related to the breast cancer therapy, and that using ≥2 cm as a measure of lymphedema may not be optimal. The 3-year risk of lymphedema reported in the AMAROS trial was 10% in patients in the ALND arm and 6% in the axillary RT arm defining lymphedema as ≥10% difference between the upper/lower/or both arms of the patients [Citation18]. In that trial, measurements were performed from baseline and onwards adding power to their measurements. Compared with the AMAROS trial, this study reports lymphedema in a cross-sectional manner but in the same range, because the objective findings indicate a baseline frequence of difference between the arms of 3–8%, and the actual findings were 16–18%, thus the ‘true’ frequence may indeed be in the range of 10%.
There is a difference between objective measurements and subjective findings, for example almost half of the patients report a degree of heaviness or swelling of ipsilateral upper or lower arm, but at the same time they do not use an arm sleeve or have objective swelling. The reason for that might be that symptoms of heaviness can also be caused by muscle weakness and sensory disturbances following surgery and systemic treatment. Danish patients are provided with a free arm sleeve if they feel heaviness or swelling of the arm even though they do not fulfil the ≥2 cm swelling criteria used in this study.
The differences between objective findings and PROM’s were also seen regarding shoulder function, since only a few patients had objective reduced abduction and flexion, although a high amount of the patients reported impairments in daily living functions. This indicates that other factors may influence the symptoms of muscular weakness including recent chemotherapy and endocrine therapy in addition to damage to axillary nerves during the ALND.
The RT technique used in our department ensures a humeral head sparing technique which is also recommended in the ESTRO consensus on target volume delineation in early breast cancer [Citation19,Citation20]. This technique compromises coverage to the most lateral PTV of axillary level 2, but causes no increased risk of regional recurrences [Citation21]. In studies of morbidity after breast cancer RT performed before the era of 3D dose planning, the shoulder joint and axilla received higher dose than with today’s treatment also causing a discrepancy between the actual expected lesser morbidity caused by modern regional nodes RT compared with the severe morbidity described previously [Citation6,Citation7].
Ideally, patients included in a morbidity study should be followed prospectively from before surgery/RT in order to report the incidence per year of relevant morbidity parameters, and also consider intervention and rehabilitation [Citation22]. That is now taking place in the Skagen Trial 1. The lack of an international consensus on the definition of arm lymphedema and the optimal time for its measurement is a major reason explaining much variation in results in older studies [Citation1]. However, also in retrospective trials, low risks of lymphedema have been reported by adding regional nodes RT to BCS and ALND [Citation23], although regional nodes RT was reported to be the only significant risk factor for arm lymphedema.
There was one-year longer follow up time in group 2 patients in this study, and since lymphedema develops slowly this may influence the results. However, from a systematic review [Citation1] it is reported that 80% of all cases of lymphedema develop within 3 years follow up, thus the likelihood of a significant difference between results obtained at 3 and 4 years is low. The primary endpoint of the study was to obtain 3-year data on lymphedema in patients treated with ALND and regional RT, and that was fulfilled.
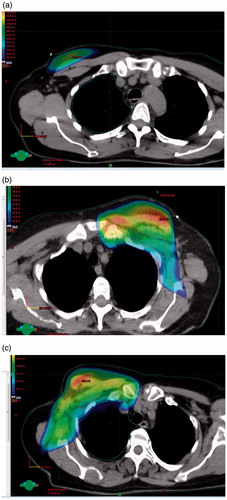
There is increasingly focus on less aggressive axillary treatment approaches [Citation24], however, the justification of regional nodes RT is still relevant, since two recent publications found improved disease free survival by adding regional nodes RT to surgery including ALND [Citation25,Citation26]. In these trials the frequencies of lymphedema were 12% and 8.4%, respectively, after regional nodes RT, but in both studies the risk of lymphedema was increased compared with the group randomized to no regional nodes RT. Also in a recent Danish population-based cohort study an overall survival benefit of internal mammary lymph nodes irradiation was found, but unfortunately morbidity evaluation was not performed prospectively in that study [Citation27]. The coverage of axillary level 1 after WBI is very sparse (), which is in harmony with other studies [Citation28]. In patients having ALND and macrometastasis to regional nodes, the regional nodes RT should not include axillary level 1() (except in cases of many involved nodes), whereas every patient with axillary metastasis and no ALND should be considered for regional nodes RT with inclusion of all axillary levels ().
Figure 2. (a) WBI: dose color wash indicating 90% isodose. (b) Loco-regional RT: axillary level 1 not included; dose color wash indicating 90% isodose. (c) Loco-regional RT: axillary level 1 included; dose color wash indicating 90% isodose.
In future treatment of early breast cancer, it is mandatory to use high quality target definition as recommended in the ESTRO consensus combined with optimal RT techniques to ensure optimal outcome.
Conclusions
The 3-year risk of lymphedema was estimated to be around 10% in patients after ALND and in this study appeared not dependent on the volume of RT (WBI or loco-regional RT). This estimate was based on bilateral objective arm measurements indicating that there is a baseline difference in the arms irrespective of breast cancer therapy, which was accounted for. Impairment of shoulder function was rare and not influenced by regional nodes RT. Awareness of morbidity is important to improve future treatment strategies in heavily treated breast cancer patients. The Skagen trial 1 is open for accrual the next years where patients will undergo prospective morbidity evaluation, and this will help us understand more the biology of late morbidity.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Additional information
Funding
References
- DiSipio T, Rye S, Newman B, et al. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14:500–515.
- Gartner R, Jensen MB, Kronborg L, et al. Self-reported arm-lymphedema and functional impairment after breast cancer treatment – a nationwide study of prevalence and associated factors. The Breast. 2010;19:506–515.
- Tsai RJ, Dennis LK, Lynch CF, et al. The risk of developing arm lymphedema among breast cancer survivors: A meta-analysis of treatment factors. Ann Surg Oncol. 2009;16:1959–1972.
- Kissin MW, Querci della Rovere G, Easton D, et al. Risk of lymphedema following the treatment of breast cancer. Br J Surg. 1986;73:580–584.
- Overgaard M, Bentzen SM, Christensen JJ, et al. The value of the NSD formula in equation of acute and late radiation complications in normal tissue following 2 and 5 fractions per week in breast cancer patients treated with postmastectomy irradiation. Radiother Oncol. 1987;9:1–11.
- Johansen J, Overgaard J, Blichert-Toft M, et al. Treatment of morbidity associated with the management of the axilla in breast-conserving therapy. Acta Oncol. 2000;39:349–354.
- Højris I, Andersen J, Overgaard M, et al. Late treatment-related morbidity in breast cancer patients randomized to postmastectomy radiotherapy and systemic treatment versus systemic treatment alone. Acta Oncol. 2000;39:355–372.
- Lauridsen MC, Overgaard M, Overgaard J, et al. Shoulder disability and late symptoms following surgery for early breast cancer. Acta Oncol. 2008;47:569–575.
- Madsen AH, Haugaard K, Sørensen J, et al. Arm morbidity following sentinel lymph node biopsy or axillary lymph node dissection: A study from the Danish Breast Cancer Cooperative Group. Breast. 2008;17:138–147.
- Offersen BV, Jacobsen EH, Nielsen MH, et al. on behalf of the DBCG RT Committee. First results from the clinically controlled randomised DBCG HYPO trial, Late breaking abstract. 2016:E35–2532.
- Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362:513–520.
- Bentzen SM, Agrawal RK, Aird EG, et al. The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet Oncol. 2008;9:331–341.
- Bentzen SM, Agrawal RK, Aird EGA, et al. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet. 2008;371:1098–1107.
- Haviland JS, Owen JR, Dewar JA, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14:1086–1094.
- Hopwood P, Fletcher I, Lee A, et al. A body image scale for use with cancer patients. Eur J Cancer. 2001;37:189–197.
- Lyngholm CD, Christiansen PM, Damsgaard TE, et al. Long-term follow-up of late morbidity, cosmetic outcome and body image after breast conserving therapy. A study from the Danish Breast Cancer Cooperative Group (DBCG). Acta Oncol. 2013;52:259–269.
- Thorsen LB, Thomsen MS, Berg M, Danish Breast Cancer Cooperative Group Radiotherapy committee, et al. CT-planned internal mammary node radiotherapy in the DBCG-IMN study: benefit versus potentially harmful effects. Acta Oncol. 2014;53:1027–1034.
- Donker M, van Tienhoven G, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014;15:1303–1310.
- Offersen BV, Boersma LJ, Kirkove C, et al. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer. Radiother Oncol. 2015;114:3–10.
- Offersen BV, Boersma LJ, Kirkove C, et al. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer, version 1.1. Radiother Oncol. 2016;118:205–208.
- Nielsen HM, Offersen BV. Regional recurrence after adjuvant breast cancer radiotherapy is not due to insufficient target coverage. Radiother Oncol. 2015;114:1–2.
- Ewertz M, Jensen AB. Late effects of breast cancer treatment and potentials for rehabilitation. Acta Oncol. 2011;50:187–193.
- Powell SN, Taghian AG, Kachnic LA, et al. Risk of lymphedema after regional nodal irradiation with breast conservation therapy. Int J Radiat Oncol Biol Phys. 2003;55:1209–1215.
- Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillarydissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305:569–575.
- Poortmans PM, Collette S, Kirkove C, EORTC Radiation Oncology and Breast Cancer Groups, et al. Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med. 2015;373:317–327.
- Whelan TJ, Olivotto IA, Parulekar WR, MA.20 Study Investigators, et al. Regional nodal irradiation in early-stage breast cancer. N Engl J Med. 2015;373:307–316.
- Thorsen LB, Offersen BV, Danø H, et al. DBCG-IMN: a population-based cohort study on the effect of internal mammary node irradiation in early node-positive breast cancer. J Clin Oncol. 2016;34:314–320.
- Orecchia R, Huscher A, Leonardi M, et al. Irradiation with standard tangential breast fields in patients treated with concervative surgery and sentinel node biopsy: using a three-dimensional tool to evaluate the first level coverage of the axillary nodes. BJR. 2005;78:51–54.
