Abstract
Background: Side effects of chemotherapy may occur at different time-points in the treatment cycle, and the exact assessment time relative to chemotherapy may affect HRQoL scores. The current study examined the variation of HRQoL during chemotherapy cycles, and whether differences in HRQoL scores varied at selected time-points between patients allocated to two different chemotherapy regimens.
Material and methods: Patients with stage IIIB or IV non-small-cell lung cancer (NSCLC) were randomly assigned to receive three cycles of carboplatin plus vinorelbine (VC) or gemcitabine (GC) every 3 weeks. HRQoL was reported on the EORTC QLQ-C30 and LC13 on days 1, 4, 8, 11 and 15 of every cycle. Global health status, nausea/vomiting, fatigue and dyspnea (LC13) were defined as the HRQoL scales of primary interest.
Results: Fifty-two patients were enrolled. Variation of mean scores of global health status, nausea/vomiting and fatigue showed a consistent pattern during chemotherapy. Day 4 appeared to be the time-point when chemotherapy influenced HRQoL the most. The differences in mean HRQoL scores between the two treatment arms varied at the different time-points, especially for nausea/vomiting.
Conclusion: There was a clinically relevant variation of HRQoL during chemotherapy cycles, with increased symptom burden the first week following treatment. Our results suggest that timing of HRQoL assessment can influence the chances of detecting differences between the treatment regimens.
Introduction
The most common efficacy outcomes evaluated in cancer clinical trials are overall survival and objective measures of disease control such as response rates and progression-free survival. In order to provide a patient perspective, many clinical cancer trials, including trials in non-small-cell lung cancer (NSCLC), incorporate assessment of health-related quality of life (HRQoL) [Citation1,Citation2]. HRQoL is a multidimensional construct, which covers subjective perceptions of physical, emotional, social and cognitive functions, as well as symptoms arising from the disease and side effects of cancer treatment [Citation3]. Measuring HRQoL may help patients and physicians to decide on the best treatment, based on the knowledge of the expected benefits and toxicity of therapies [Citation4].
For HRQoL data to be clinically useful, it must be acquired with appropriate instruments and methods. The choice of time-points for HRQoL assessments could be of particular importance when studying the effects of chemotherapy, which is often toxic and with a narrow therapeutic index. Assessments during treatment can either be time-based, given a set number of weeks after randomization, or event-based, coinciding with treatment cycles. Most common, HRQoL is registered at times considered convenient, i.e. when a patient comes to the clinic for a new cycle of chemotherapy. However, assessments at these time-points may not necessarily capture clinically important effects, as acute side effects of the previous cycle may no longer be present.
Only few studies have investigated the importance of timing of HRQoL assessments during chemotherapy. Retrospective analyses of clinical trial data have shown that the exact assessment time relative to chemotherapy administration may result in statistical and potentially clinically significant differences in several HRQoL domains [Citation5]. Prospective studies have found that the acute effects on HRQoL due to treatment toxicity are most severe in the first week following each course of treatment. However, these studies used simplified HRQoL instruments, such as diary cards where patients score intensity of selected symptoms [Citation6,Citation7], or included patients with a mixture of cancer types and chemotherapy regimens [Citation8].
Several previous trials comparing chemotherapy regimens in advanced NSCLC had failed to show differences in HRQoL, despite significant differences in objectively measured toxicity and a clinical impression that some regimens were better tolerated than others [Citation9–11]. Based on these experiences, we hypothesized that the timing of HRQoL assessments might influence the chances of detecting differences in HRQoL provided by different chemotherapy regimens. To explore this hypothesis, we conducted an exploratory, prospective, randomized clinical trial of patients with advanced NSCLC eligible for palliative chemotherapy. The primary aim was to assess whether there were variations of HRQoL scores during chemotherapy cycles. The secondary aim was to investigate whether differences in HRQoL scores varied at selected time-points between patients allocated to two different chemotherapy regimens.
Material and methods
Design and approval
The study was designed as an open, randomized single-center study and was approved by the Regional Committee for Medical and Health Research Ethics, Central Norway and NSD – Norwegian Center for Research Data.
Eligibility criteria and randomization
Eligible patients had NSCLC stage IIIB or IV not eligible for curative treatment, WHO performance status (PS) 0–2, no prior chemotherapy and adequate bone marrow, liver and renal function for receiving chemotherapy. After signing the informed consent form and completing the baseline HRQoL, patients were randomized to receive vinorelbine plus carboplatin (VC) or gemcitabine plus carboplatin (GC). Randomization was stratified by performance status (0–1 vs. 2), sex (male vs. female) and symptomatic brain metastases (present vs. not present).
Chemotherapy
Platinum-doublet regimens are recommended as first-line chemotherapy in advanced NSCLC. In the current study, carboplatin was combined with either vinorelbine or gemcitabine. In a trial comparing these regimens, there were no significant differences in survival or HRQoL, but more hematological toxicity from the gemcitabine regimen [Citation11]. All patients were to receive three courses of chemotherapy in 3-week cycles. Carboplatin (AUC =5, Calvert′s formula) was administered on day 1, and vinorelbine 25 mg/m2 (VC) or gemcitabine 1000 mg/m2 (GC) on days 1 and 8. Patients who were 75 years and above had a 25% dose reduction of both compounds from the first course.
All patients received prophylactic antiemetic therapy with glucocorticoids and a 5-HT-3-antagonist on days 1 and 8. Other antiemetics were allowed. Blood counts were performed before each treatment. In case of severe hematological toxicity, protocol-specified dose reductions were performed and maintained for subsequent cycles. The study treatment was discontinued in the event of disease progression, unacceptable toxicity or at the patient’s request.
Assessments
Patients were examined and underwent laboratory testing before each cycle of chemotherapy. Health-related quality of life (HRQoL) was assessed using the European Organization for Treatment of Cancer (EORTC) Quality of Life Questionnaire-Core 30 (QLQ-C30) and the lung cancer-specific module LC13 [Citation12,Citation13]. The EORTC questionnaires are among the most frequently used HRQoL instruments in cancer trials, and the psychometric properties have been extensively evaluated [Citation1,Citation14]. QLQ-C30 evaluates five functions (physical, role, cognitive, emotional and social), nine symptoms (fatigue, pain, nausea and vomiting, dyspnea, loss of appetite, insomnia, constipation, diarrhea and financial difficulties) and global health status. The LC13 questionnaire measures symptoms commonly associated with lung cancer and its treatment (dyspnea, coughing, hemoptysis, sore mouth, dysphagia, peripheral neuropathy, alopecia, pain in chest, pain in arm or shoulder and pain in other parts).
HRQoL questionnaires (on paper) were completed on days 1, 4, 8, 11 and 15 of every 3-week cycle of chemotherapy. On days 1, 8 and 15 of each cycle, the questionnaires were completed at the hospital when the patients came for infusion of chemotherapy (days 1 and 8) or blood tests (day 15). Patients completed the questionnaires on days 4 and 11 at home. The questionnaires were handed to the patients at the hospital visits on days 1 and 8 along with instructions on when to complete the questionnaires. The questionnaires were returned at the next hospital appointment. To avoid overlapping assessment intervals, the recall period on the questionnaires was changed from ‘the last week’ to ‘the last three days’. Since the primary interest in this study was the variation of HRQoL during a cycle of chemotherapy, the HRQoL assessments were only performed as long as the patients received chemotherapy.
Statistical considerations
Primary endpoint was to assess the intra-cycle variation in HRQoL scores during chemotherapy. Secondary endpoint was to assess the differences in HRQoL scores between treatment arms at selected time-points in the treatment cycle. The primary HRQoL outcomes were defined as global health status, nausea/vomiting, fatigue and dyspnea (LC13), since these were defined as the clinically most relevant in previous studies comparing first-line regimens in advanced NSCLC [Citation9–11]. Global health status gives information on the patient′s overall health status, dyspnea and fatigue are frequent symptoms in lung cancer, and fatigue and nausea/vomiting are common side effects of chemotherapy. In addition, as exploratory analyses, we reported changes in the other HRQoL scales. The objective of the study was to demonstrate ‘proof of principle’ rather than conduct a fully powered HRQoL comparison of the two treatment regimens. Since no similar studies had been conducted, there were no data available for a sample size calculation. We estimated that 25 patients in each treatment group would be sufficient to provide an indication of whether there were clinically relevant variations in HRQoL scores during chemotherapy cycles.
All domain scores of QLQ-C30 and LC13 were linearly transformed into a scale from 0 to 100 according to the EORTC QLQ-C30 scoring manual [Citation15]. A high score in global health status or a functional scale represent good health status or high level of functioning, while a high symptom scale score represents more symptoms. A mean change of 5–10 points has been reported to correspond to ‘a little change’, a change of 10–20 points to ‘a moderate change’ and a change of more than 20 points to ‘a large change’ [Citation16].
The primary analysis was to compare mean HRQoL scores of reported values at each time-point. To explore potential differences in HRQoL scores over time between the two treatment arms, we also applied a linear mixed model for longitudinal analyses. The baseline scores, treatment arm, time (as a categorical variable) and treatment-by-time interaction were included as fixed effects. The model included a random intercept at patient level, and employed a first-order autoregressive covariance structure. The purpose of the analyses was to estimate effect sizes and p values or confidence intervals were not reported. Sensitivity analyses were performed including only questionnaires completed as scheduled (plus/minus one day). For all analyses, we used Stata version 13.1 (College Station, TX, USA).
Results
Characteristics of the patients
Between September 2009 and May 2012, 52 patients were randomized to receive VC (n = 25) or GC (n = 27). The study arms were well balanced with respect to demographic and clinical characteristics (). Forty-one patients completed all three cycles (VC: 68%, GC: 89%). The mean number of cycles was 2.6 in the VC arm and 2.9 in the GC arm.
Table 1. Patient characteristics at baseline and chemotherapy completion.
HRQoL completion rates
The patients completed 693 of the 756 HRQoL questionnaires (92%) during the three cycles of chemotherapy. Compliance was highest at the start of each cycle (94% to 100%), and decreased during each cycle period (). The completion rate was lower at days 1, 15 and 22 in cycle 3 in the VC group (VC: 65%, GC: 96%). Otherwise, the completion rates were comparable in the two groups. Overall, 620 questionnaires (88%) were completed as scheduled (plus/minus one day). The assessments deviating from the planned schedule were mainly (68%) due to delayed administration of chemotherapy on day 8 in cycle 2 or 3, which lead to a delayed completion of questionnaires on days 8, 11 and 15 in the same cycle.
Table 2. Completion rates of HRQoL questionnaires.
Variation of HRQoL during chemotherapy
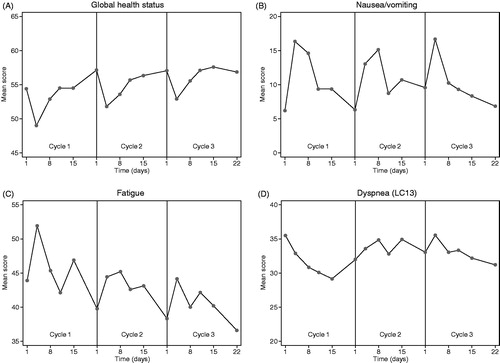
shows the variation in raw mean scores for primary HRQoL scales during the three cycles of chemotherapy. In cycle 1, global health status decreased after the first day of chemotherapy, with the lowest score registered at day 4 (5.4 points decrease from day 1). Nausea/vomiting and fatigue symptoms peaked around day 4 (10.1 and 8.0 points increase from day 1, respectively). In all these scales, scores were worse during the first week following treatment and resolved before the next cycle. The same patterns were observed in the second and third treatment cycle, although the changes were smaller. The severity of dyspnea (LC13) decreased gradually during the first cycle, but this pattern did not reproduce in the second and third treatment cycle. Overall, the mean score for dyspnea (LC13) was not related to time-point in the chemotherapy cycle. The plots of the scores of only the questionnaires completed on schedule (plus/minus one day) showed similar results (data not shown).
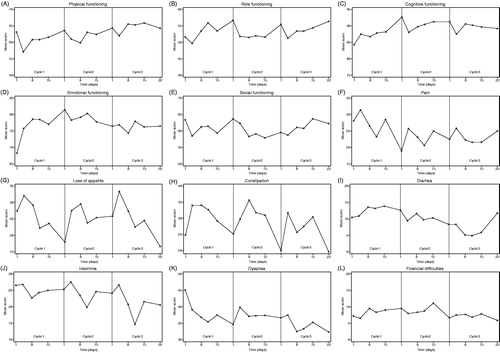
In the remaining HRQoL scales, the following showed a consistent pattern of repetitive intra-cycle changes: appetite loss (highest symptom score at day 4 and 8), constipation (highest symptom score day 4, 8 and 15) and insomnia (lowest symptom score on day 11) ( and ).
Comparison of HRQoL between treatment arms
and show the mean scores over time for primary HRQoL scales by treatment arm. For each scale, there are two plots: The raw mean scores and the estimated mean scores from the linear mixed model. The raw profiles show that the HRQoL scores at baseline were well balanced across the two treatment groups with a difference in mean scores of <5 points, except dyspnea (LC13) with a 7.8 points higher mean score in the GC arm. For global health status, comparison of mean scores favored VC with the largest differences found on day 11 (7.3, 7.2 and 16.4 points in cycle 1, 2 and 3, respectively) and day 15 (4.6, 11.6 and 15.6 points in cycle 1, 2 and 3, respectively). For nausea/vomiting, the difference between the treatment arms ranged from 5.6 points favoring VC to 14.0 points favoring GC. The largest differences were seen at day 4, when patients in the VC arm had more nausea/vomiting (mean difference 8.3, 14.0 and 5.2 points in cycle 1, 2 and 3, respectively). In the fatigue scale, the differences ranged from 4.2 points favoring GC to 7.6 points favoring VC, but there was no repetitive pattern of cycle-specific differences. In the dyspnea (LC13) scale, the baseline difference between the treatment arms depicted in the raw score plot, decreased during the treatment period.
Figure 4. Mean raw scores and linear mixed model estimates for global health status and nausea/vomiting scales over time (by treatment arm). VC: vinorelbine + carboplatin; GC: gemcitabine + carboplatin.
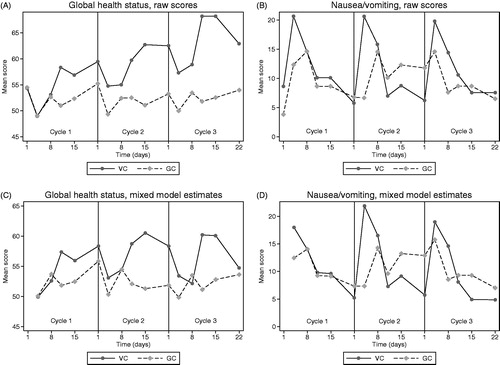
Figure 5. Mean raw scores and linear mixed model estimates for fatigue and dyspnea (LC13) scales over time (by treatment arm). VC: vinorelbine + carboplatin; GC: gemcitabine + carboplatin.
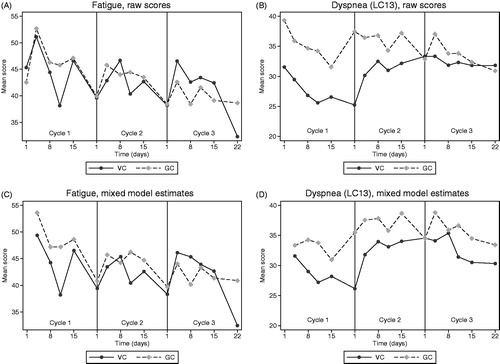
The same patterns of differences in global health status and nausea/vomiting were observed in plots of estimated means from the linear mixed model. Especially in cycle 3, the difference between the treatment arms was smaller (). Sensitivity analyses including only the questionnaires completed on the scheduled dates plus/minus one day gave similar results and conclusions (data not shown).
Discussion
The results of the current study elucidate several issues concerning timing of HRQoL assessments in cancer trials. First, frequent assessments using a comprehensive HRQoL instrument are feasible, even in a population of advanced cancer patients. It has been suggested that a completion rate greater than 80% is acceptable [Citation17]. The overall completion rate in this study was 92%, and higher than 80% at all time-points except the last assessments in cycle 3 in the VC arm. Second, this study found a consistent pattern of variation in several HRQoL scales during the chemotherapy cycles, indicating that the scores are influenced by the timing of measurements relative to treatment. The variation in HRQoL was most pronounced during cycle one. This might be explained by more symptomatic treatment or dose reductions in cycle 2 and 3 in those patients who experienced most toxicity, or even discontinuation of chemotherapy (11 of the 52 patients did not complete three cycles of chemotherapy). There was, however, a pattern of transient worsening also in cycles 2 and 3. Third, the timing of assessments can potentially influence comparison of treatments, since differences in some HRQoL scales varied during the chemotherapy cycles.
The first studies to examine variation of HRQoL during treatment used short and simple questionnaires. In the 1980s, the UK Medical Research Council designed a diary card for daily quality of life assessment in cancer clinical trials [Citation6]. It was decided that five or six questions would be the maximum that could reasonably be asked on a daily basis. Results from several clinical trials using such diary cards demonstrated transient changes in HRQoL occurring during cancer therapy. Often, these changes disappeared by the time of the next clinic attendance and tended not to be recorded on the clinicians´ assessment [Citation6,Citation18]. More recently, an Austrian study used the EORTC QLQ-C30 to assess HRQoL at the day of chemotherapy administration, one week later and (for some patients) two weeks later [Citation8]. Fifty-four patients were included with a wide variety of diagnoses, treated with various chemotherapy regimens with curative, adjuvant or palliative intent. The study found increased symptom burden one week after chemotherapy administration in 9 of the 15 domains of the EORTC QLQ-C30, with the largest differences in fatigue, constipation and appetite loss. We consider these results to be well in line with the findings in our study, which, to our knowledge, is the largest cancer study that has measured HRQoL this frequently using a comprehensive questionnaire.
The main limitation of the current study is the small sample size, though we believe that the number of patients was sufficient for an exploratory study. Although we consider completion rates to be high, there was more missing data towards the end of each cycle. In the VC arm, fewer patients received three cycles of chemotherapy and the completion rates were lower at the last assessments. The reason for the lower completion rate is unknown, since the study was not designed to assess reasons for non-completion of the questionnaires. Other studies have shown that patients with a poor health status may be less likely to complete HRQoL questionnaires, which might influence the results [Citation19]. There is no standard method for dealing with missing values in HRQoL analyses, but approaches such as linear mixed modeling may provide more robust estimates of treatment effects. For explorative purposes, we used a shorter recall period of the EORTC questionnaires than in the validated versions (three instead of seven days). Failure to complete all questionnaires as scheduled might have influenced the results. However, 88% of questionnaires were completed according to schedule (plus/minus one day), and sensitivity analyses of only the questionnaires completed on schedule showed similar results as the primary analyses.
Strengths of the study are the homogenous patient sample, rigorous data collection throughout the study period and high completion rates of questionnaires. Baseline HRQoL scores were comparable to former trials of first-line chemotherapy in advanced NSCLC [Citation9–11], and the chemotherapy regimens administered are established treatment options.
In a systematic review of HRQoL reporting in randomized controlled trials in NSCLC, more than half of the trials revealed significant differences in HRQoL scores [Citation1]. However, in several of these trials, there was not a consistent pattern of HRQoL benefit for any regimen. Thus, it has been questioned whether HRQoL data constitute enough evidence to make treatment recommendations [Citation2]. Forty-five trials in the systematic review reported details about the timing of assessments relative to chemotherapy administration [Citation1]. In most of these trials, HRQoL was assessed at the end of a chemotherapy cycle or immediately before the start of the next cycle, or at fixed time-points corresponding to cycle length, but regardless of modifications of the treatment schedule. Only four trials (9%) measured HRQoL at several time-points in the chemotherapy cycles, either with weekly assessments [Citation20,Citation21] or with an assessment at day 8 in cycle 1 in addition to assessments at the end of each 3-week cycle [Citation22,Citation23]. Compared to the end of cycle assessments, scores in week one assessments were worse in several symptoms and with more pronounced differences between treatment arms. Thus, suboptimal timing of HRQoL assessments and too few measurements might be the reason for the relatively little impact of HRQoL studies on treatment recommendations in cancer care.
Taking the timing of measurements into account can improve the understanding of benefits and toxicity of therapies, highlight time-dependent differences and result in more correct and balanced conclusions about treatment effects. The current study compared two regimens with similar schedules and carboplatin doses, found to be equally effective and tolerable in a previous study [Citation11]. When assessing HRQoL repeatedly in the present study, we found that patients in the VC arm tended to have better mean scores for global health status during the last part of chemotherapy cycles, but worse scores for nausea/vomiting in the first week following treatment. This illustrates the need for clinical trials not only to select the outcomes of interest, but also the appropriate time to measure them. Due to different toxicities and schedules of the drug(s) in use, HRQoL profiles during treatment might be specific for each regimen. Ideally, when the HRQoL trajectory is unknown, frequent assessments in a small number of patients should be considered for all regimens before embarking on a larger, comparative, intervention study.
Conclusion
In conclusion, the timing of HRQoL assessment during chemotherapy may affect HRQoL scores and potentially influence the chances of detecting HRQoL differences between treatment regimens. Assessment schedules based on the symptom trajectory can improve validity of trials, by providing more accurate information about the HRQoL experienced by the patients. In the clinical context, increased understanding of patients’ symptom burden during chemotherapy cycles may help improve supportive care and thus the deliverance of anticancer treatment.
Disclosure statement
The authors declare no conflict of interest.
References
- Claassens L, van Meerbeeck J, Coens C, et al. Health-related quality of life in non-small-cell lung cancer: an update of a systematic review on methodologic issues in randomized controlled trials. J Clin Oncol. 2011;29:2104–2120.
- Saad ED, Adamowicz K, Katz A, et al. Assessment of quality of life in advanced non-small-cell lung cancer: an overview of recent randomized trials. Cancer Treat Rev. 2012;38:807–814.
- Bottomley A. The cancer patient and quality of life. Oncologist. 2002;7:120–125.
- Au HJ, Ringash J, Brundage M, et al. Added value of health-related quality of life measurement in cancer clinical trials: the experience of the NCIC CTG. Expert Rev Pharmacoecon Outcomes Res. 2010;10:119–128.
- Ediebah DE, Coens C, Maringwa JT, et al. Effect of completion-time windows in the analysis of health-related quality of life outcomes in cancer patients. Ann Oncol. 2013;24:231–237.
- Fayers P. MRC quality of life studies using a daily diary card-practical lessons learned from cancer trials. Qual Life Res. 1995;4:343–352.
- Rudd RM, Gower NH, Spiro SG, et al. Gemcitabine plus carboplatin versus mitomycin, ifosfamide, and cisplatin in patients with stage IIIB or IV non-small-cell lung cancer: a phase III randomized study of the London Lung Cancer Group. J Clin Oncol. 2005;23:142–153.
- Giesinger JM, Wintner LM, Zabernigg A, et al. Assessing quality of life on the day of chemotherapy administration underestimates patients’ true symptom burden. BMC Cancer. 2014;14:758.
- Flotten O, Gronberg BH, Bremnes R, et al. Vinorelbine and gemcitabine vs vinorelbine and carboplatin as first-line treatment of advanced NSCLC. A phase III randomised controlled trial by the Norwegian Lung Cancer Study Group. Br J Cancer. 2012;107:442–447.
- Gronberg BH, Bremnes RM, Flotten O, et al. Phase III study by the Norwegian lung cancer study group: pemetrexed plus carboplatin compared with gemcitabine plus carboplatin as first-line chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2009;27:3217–3224.
- Helbekkmo N, Sundstrom SH, Aasebo U, et al. Vinorelbine/carboplatin vs gemcitabine/carboplatin in advanced NSCLC shows similar efficacy, but different impact of toxicity. Br J Cancer. 2007;97:283–289.
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376.
- Bergman B, Aaronson NK, Ahmedzai S, et al. The EORTC QLQ-LC13: a modular supplement to the EORTC core quality of life questionnaire (QLQ-C30) for use in lung cancer clinical trials. EORTC Study Group on Quality of Life. Eur J Cancer. 1994;30a:635–642.
- Damm K, Roeske N, Jacob C. Health-related quality of life questionnaires in lung cancer trials: a systematic literature review. Health Econ Rev. 2013;3:15.
- Fayers PM, Aaronson N, Bjordal K. EORTC QLQ-C30 Scoring Manual. 3rd ed. Brussels: European Organisation for Research and Treatment of Cancer; 2001.
- Osoba D, Rodrigues G, Myles J, et al. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–144.
- Fayers P, Machin D. Quality of life: the assessment, analysis and interpretation of patient-reported outcomes. Chichester: John Wiley & Sons; 2007.
- Fayers PM, Bleehen NM, Girling DJ, et al. Assessment of quality of life in small-cell lung cancer using a daily diary card developed by the Medical Research Council lung cancer working party. Br J Cancer. 1991;64:299–306.
- Curran D, Molenberghs G, Fayers PM, et al. Incomplete quality of life data in randomized trials: missing forms. Statist Med. 1998;17:697–709.
- Vansteenkiste JF, Vandebroek JE, Nackaerts KL, et al. Clinical-benefit response in advanced non-small-cell lung cancer: a multicentre prospective randomised phase III study of single agent gemcitabine versus cisplatin-vindesine. Ann Oncol. 2001;12:1221–1230.
- Crino L, Cappuzzo F, Zatloukal P, et al. Gefitinib versus vinorelbine in chemotherapy-naive elderly patients with advanced non-small-cell lung cancer (INVITE): a randomized, phase II study. J Clin Oncol. 2008;26:4253–4260.
- Gridelli C, Gallo C, Shepherd FA, et al. Gemcitabine plus vinorelbine compared with cisplatin plus vinorelbine or cisplatin plus gemcitabine for advanced non-small-cell lung cancer: a phase III trial of the Italian GEMVIN Investigators and the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2003;21:3025–3034.
- Gridelli C, Gallo C, Ceribelli A, et al. Factorial phase III randomised trial of rofecoxib and prolonged constant infusion of gemcitabine in advanced non-small-cell lung cancer: the GEmcitabine-COxib in NSCLC (GECO) study. Lancet Oncol. 2007;8:500–512.