Abstract
Background: Radiation-induced cognitive impairment may be mediated by hippocampal damage, but the structural integrity of this region in tumor patients at baseline is unclear. Hippocampal volumes of 31 glioma patients prior to receiving radiotherapy were compared to a group of 34 healthy controls.
Materials and methods: Left and right hippocampi on T1-weighted pre-contrast magnetic resonance images were automatically segmented using Freesurfer, and visually inspected for segmentation errors. Normalized hippocampal volume for each subject was calculated as the sum of left and right hippocampal volumes divided by the estimated total intracranial volume. The normalized amygdala volume was similarly analyzed as a reference structure.
Results: A Wilcoxon rank-sum test showed a significant difference in normalized hippocampal volumes between patients and controls (mean value 0.499 vs. 0.524, p = .01). No statistically significant difference was found for the amygdala. A post-hoc analysis revealed a significant difference in normalized hippocampal volumes between patients who had experienced seizures (mean value: 0.480, p < .05) and controls. No difference was noted between patients without seizures (mean value: 0.513) and controls.
Conclusions: Hippocampi of glioma patients prior to radiotherapy were significantly smaller than those of age-matched controls. Group differences were larger in patients with tumor-associated seizures. This may be secondary to other processes such as tumor biology and inflammation.
Introduction
Radiation therapy (RT) is a mainstay in the treatment of malignant brain tumors [Citation1]. Substantial advancements in treatment planning now allow for precise control over delivered dose distributions, with increasing attention paid to sparing radiation-sensitive normal tissue [Citation2]. In recent years, the effect of radiation on the hippocampus has gained particular interest [Citation3]. Hippocampal-sparing RT techniques are being evaluated in nationwide clinical trials (RTOG 0933) [Citation4] as a means to mitigate post-RT neurocognitive sequelae, particularly with respect to learning and memory.
Interest in the hippocampus has been driven by several studies showing macro- and micro-structural changes post-RT in pre-clinical models [Citation5,Citation6] and retrospective analyses demonstrating an association between cognitive impairment and hippocampal irradiation [Citation7]. However, there has been little work done to assess the structural integrity of the hippocampus in these patients prior to RT. Other work has suggested that tumor biology [Citation8], inflammation [Citation9] and chemotherapy [Citation10] may have detrimental impact on hippocampal microstructure, which may affect brain tumor patients even before they receive RT. Patients may, therefore, have damaged or structurally compromised hippocampi at the outset of RT.
In this study, we sought to determine if the hippocampal volumes of brain tumor patients prior to RT differed from those of age- and gender-matched healthy individuals. Hippocampal volume, as measured using volumetric MRI, was used as an imaging biomarker of structural integrity due to its extensive use in the neuroimaging literature [Citation11]. We also sought to examine whether any important clinical variables, such as clinical seizure history, could account for baseline differences in hippocampal volumes.
Materials and methods
Patients
From January 2011 to December 2013, 88 patients were treated with fractionated brain RT for primary brain tumors at the University of California San Diego Moores Cancer Center. To meet inclusion criteria, patients must have had a high-resolution T1 pre-contrast volumetric MRI prior to RT. Patients were excluded if T1-weighted contrast enhancement (suggesting tumor) or T2-weighted FLAIR hyper-intensity (suggesting edema) extended to either the left or right hippocampus. A control group was selected from a group of healthy individuals with ages spanning the range of the patient group. A total of 31 patients and 34 controls were selected for this study. This study was approved by the institutional review board.
MR image acquisition and processing
MR imaging was performed on a 3T Signa Excite HDx scanner (GE Healthcare, Milwaukee, WI) equipped with an 8-channel head coil. The imaging protocol for brain tumor patients included pre- and post-contrast 3D volumetric T1-weighted sequence (TE = 2.8 ms, TR = 6.5 ms, TI = 450 ms). Imaging of healthy controls was collected on a 3T Discovery MR750 (GE Healthcare, Milwaukee, WI) scanner with an 8-channel phased-array head coil. This image acquisition included a T1-weighted 3D structural scan (TE = 3.16 ms, TR = 8.08 ms, TI = 600 ms). Patient and control image data were corrected for gradient non-linearities and resampled to a common atlas space (1 mm isotropic voxels) using in-house algorithms developed in MATLAB (MathWorks, Natick, MA, USA).
Normalized hippocampal volume
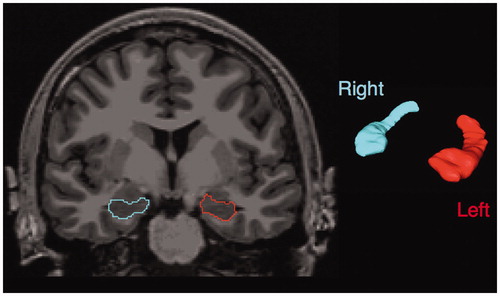
Patient and control hippocampi were segmented using the automated FreeSurfer processing pipeline (version 5.3; available at http://surfer.nmr.harvard.edu), available on the Neuroscience Gateway Portal [Citation12]. T1-weighted images were used as the input structural volume. Segmentations were visually inspected for accuracy. An example of the segmentation provided by Freesurfer is shown in . The left, right and estimated total intracranial volumes, as determined by FreeSurfer, were recorded for each individual. The absolute hippocampal volume was calculated as the sum of left and right hippocampi. The normalized hippocampal volume was calculated as the absolute hippocampal volume divided by the estimated total intracranial volume and multiplied by 100% [Citation13]. The normalized amygdala volume was similarly calculated to determine if any volumetric changes detected were specific to the hippocampus [Citation14]. The amygdala was chosen as it is an adjacent limbic structure that is often used for this purpose within the neuroimaging literature [Citation14].
Statistical analyses
Wilcoxon’s rank-tests were used to compare distributions of absolute hippocampal volume, normalized hippocampal volume, normalized amygdala volume, age and estimated total intracranial volume between the patient and control group. A Fisher’s exact test was used to compare the distribution of males and females between the control and patient group. The subjects were further divided by seizure status (controls, patients without seizures, patients with seizures), as determined by clinical evidence of seizures, of any classification, as an initial presenting symptom. The effect of seizure status on normalized hippocampal volume was assessed using a Kruskal–Wallis test. The significance level, alpha, was set at .05. All statistical analyses were performed using R environment for statistical computing v3.1.3.
Results
Group characteristics – age, sex and intracranial volume
A summary of group characteristics is shown in . Wilcoxon’s rank-sum tests showed that patient and control groups did not differ significantly in age. Fisher’s exact test showed that there was not a significant difference in the male to female ratio between patients and controls. The intracranial volume did not differ significantly between control and patient groups with mean values (± standard deviation) of 1521 cm3 (± 135) and 1533 cm3 (±111), respectively.
Table 1. Patient and control group characteristics.
Absolute hippocampal volume
The mean values of absolute hippocampal volume for controls and patients were calculated as 7.95 cm3 (±0.68) and 7.63 cm3 (±0.61), respectively. The difference in distributions between the two groups was not, however, found to be statistically significant (p = .09) using a Wilcoxon rank-sum test. When stratified by gender, the absolute hippocampal value differed significantly between controls and patient groups for males – p = .01, mean control: 8.29 cm3 (±0.69), mean patient: 7.72 cm3 (±0.62) – but not for females – p = .22, mean control: 7.58 (±0.45), mean patient: 7.31 (±0.46).
Normalized hippocampal volume
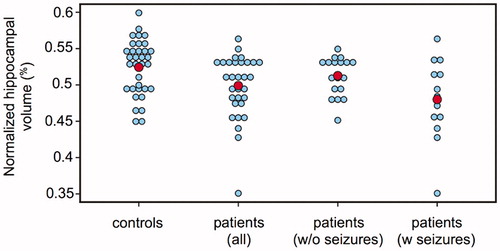
Normalized hippocampal volumes differed significantly (p = .01) between control and patient groups with mean values of 0.524% (±0.038) and 0.499% (±0.044), respectively. Dot plots of the distributions in each group are shown in . By comparison, the normalized amygdala volumes did not differ significantly between the control and patient groups, with mean values of 0.207% (±0.018) and 0.204% (±0.026), respectively
Figure 2. Dot plots of normalized hippocampal volume (unitless) in controls, all patients, patients without (w/o) seizures and patients with (w) seizures. The red dots indicate the mean value of the group. Significant differences were found between all patients vs. controls, and patients with seizures vs. controls, but not between patients without seizures vs. controls, or patients with seizures vs. patients without seizures.
Effect of seizure status
A Kruskal–Wallis test showed a statistically significant difference in normalized hippocampal volume between the various seizure status groups (adjusted p = .039) with mean values of 0.524 (±0.038) for controls, 0.513 (±0.027) for patients without seizures and 0.480 (±0.056) for patients with seizures – . The distribution of normalized hippocampal volumes differed significantly between the controls and patients with seizures (difference in mean ranks: 17.08, least significant difference: 11.55), but not between the controls and patients without seizures (difference in mean ranks: 6.99, least significant difference: 10.32). No significant difference was detected between patients with or without seizures (difference in mean ranks: 10.09, least significant difference: 12.89). A Wilcoxon-rank sum test between the normalized hippocampal volume of patients with and without seizures confirmed this (p = .09).
Discussion
Our study demonstrates that normalized hippocampal volumes of glioma patients prior to RT were significantly smaller than those of age-matched controls. The fact that neither intracranial volume nor normalized amygdala volume significantly differed between the two groups leads us to believe that we are observing an effect that is particular to the hippocampus. However, the effect of the tumor on the Freesurfer estimate of total intracranial volume has not been studied, and thus may potentiate some of the findings. Although a statistically significant difference was not found between absolute hippocampal volumes (without normalization), the marginal p value (p = .09) may indicate that we lack sufficient power to detect the difference at a significance level of 0.05. In addition, significant differences in absolute hippocampal volume were observed in males, but not females. While the present study does not reveal the mechanism of the observed atrophy, it does reveal that tissue changes within the hippocampus may already be underway in glioma patients before RT.
There are several potential causes for smaller hippocampi measured in the patient group, including surgery, tumor biology and medications. Glioma cells, particularly of astrocytic origin, may release abnormal amounts of the neurotransmitter glutamate into the extracellular space [Citation8]. Imbalances in extracellular glutamate are associated with hippocampal neuronal death in vitro [Citation8]. In addition, dexamethasone (a glucocorticoid receptor agonist commonly prescribed to treat edema in brain tumor patients, including 74% of patients in this study) is associated with disrupted hippocampal neurogenesis in pre-clinical models [Citation15]. Inflammation, potentially arising from the tumor, surgery or stress and mediated by sustained microglial activation has also been shown to impair basal hippocampal neurogenesis in rats [Citation16].
A post-hoc analysis revealed that seizure status may underlie the difference in hippocampal volume between patients and controls. Evidence suggests that seizures are associated with damage to brain tissue. A longitudinal study reported a decrease in ipsilateral hippocampal volume of patients with mild temporal lobe epilepsy, where volume loss correlated with the number of generalized seizures between scans [Citation17]. However, there has been no work, to-date, directly linking tumor-associated seizures with hippocampal atrophy. Interpretation of post-hoc analyses of modest sample sizes must be made cautiously. Nonetheless, some of the mechanisms postulated to be responsible for tumor-associated seizures (glutamate imbalances, disrupted blood–brain barrier [Citation18]) are those previously described to be associated with hippocampal neuronal changes. Although no statistically significant difference was observed between patients with and without seizures, the marginal p value obtained from the Wilcoxon rank sum test suggests a trend that warrants further investigation in a larger sample.
This study has several limitations. While the specific devices used for patient and control groups differed, both were GE scanners operated using a field strength of 3T. Previous studies have shown that scanner platform (such as Siemens vs. GE) and field strength (1.5 vs. 3T) have the strongest influence on volume measurement bias [Citation19]. In addition, the absence of statistically significant difference in estimated total intracranial volume and normalized amygdala volume gives us further reason to believe that no systematic bias was introduced to the findings as a result of scanner differences. In addition, the sample size is small. The findings will therefore need to be replicated in a larger study. The small sample size also means that covariates of potential interest such as medication type and dosage, seizure type (e.g., generalized vs. partial), seizure by gender, tumor genetic profile and tumor location could not be adequately investigated. Larger studies using prospectively collected data could best explore the potential effects of these covariates. The clinical implications of hippocampal volume loss in pre-RT brain tumor patients remain unclear, but may be better elucidated through long-term imaging and neurocognitive monitoring.
In summary, the normalized hippocampal volume of glioma patients prior to RT was smaller than healthy age-matched controls. This effect is more readily observed in patients with tumor-associated seizures. Future studies to validate the findings in larger cohorts, as well as correlating hippocampal status prior to RT with subsequent damage and cognitive dysfunction are currently underway at our institution.
Disclosure statement
T.M.S. and J.H.G. report grants from Varian Medical Systems, during the conduct of the study. A.M.D reports that he is a founder of and holds equity in CorTechs Labs Inc. and serves on its Scientific Advisory Board. He is a member of the Scientific Advisory Board of Human Longevity Inc., and receives funding through research agreements with General Electric Healthcare and Medtronic Inc.
Additional information
Funding
References
- Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370:709–722.
- Lawrence YR, Li XA, el Naqa I, et al. Radiation dose-volume effects in the brain. Int J Radiat Oncol Biol Phys. 2010;76:S20–S27.
- Gondi V, Hermann BP, Mehta MP, et al. Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int J Radiat Oncol Biol Phys. 2013;83:e487–e493.
- RTOG. A phase II trial of hippocampal avoidance during whole brain radiotherapy for brain metastases – RTOG CCOP study tle. RTOG 0933 Protocol Information; 2011.
- Peißner W, Kocher M, Treuer H, et al. Ionizing radiation-induced apoptosis of proliferating stem cells in the dentate gyrus of the adult rat hippocampus. Brain Res Mol Brain Res. 1999;71:61–68.
- Nagai R, Tsunoda S, Hori Y, et al. Selective vulnerability to radiation in the hippocampal dentate granule cells. Surg Neurol. 2000;53:503–507.
- Gondi V, Tomé WA, Mehta MP. Why avoid the hippocampus? A comprehensive review. Radiother Oncol. 2010;97:370–376.
- Ye ZC, Sontheimer H. Glioma cells release excitotoxic concentrations of glutamate. Cancer Res. 1999;59:4383–4391.
- Liu X, Wu Z, Hayashi Y, et al. Age-dependent neuroinflammatory responses and deficits in long-term potentiation in the hippocampus during systemic inflammation. Neuroscience. 2012;216:133–142.
- Christie L-A, Acharya MM, Parihar VK, et al. Impaired cognitive function and hippocampal neurogenesis following cancer chemotherapy. Clin Cancer Res [Internet]. 2012;18:1954–1965.
- Teicher MH, Anderson CM, Polcari A. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc Natl Acad Sci USA. 2012;109:E563–E572.
- Sivagnanam S, Majumdar A, Yoshimoto K, et al. Introducing the neuroscience gateway. CEUR Workshop Proc. 2013;993.
- Whitwell JL, Crum WR, Watt HC, et al. Normalization of cerebral volumes by use of intracranial volume: implications for longitudinal quantitative MR imaging. Am J Neuroradiol. 2001;22:1483–1489.
- Bremner JD, Narayan M, Anderson ER, et al. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157:115–118.
- Bin KJ, Ju JY, Kim JH, et al. Dexamethasone inhibits proliferation of adult hippocampal neurogenesis in vivo and in vitro. Brain Res. 2004;1027:1–10.
- Ekdahl CT, Claasen J-H, Bonde S, et al. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci USA. 2003;100:13632–13637.
- Briellmann RS, Berkovic SF, Syngeniotis A, et al. Seizure-associated hippocampal volume loss: a longitudinal magnetic resonance study of temporal lobe epilepsy. Ann Neurol. 2002;51:641–644.
- Shamji MF, Fric-Shamji EC, Benoit BG. Brain tumors and epilepsy: pathophysiology of peritumoral changes. Neurosurg Rev. 2009;32:274–284.
- Jovicich J, Czanner S, Han X, et al. MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. Neuroimage. 2009;46:177–192.