Abstract
Background: Only a few studies of limited size have examined whether oncoplastic breast surgery delays the onset of adjuvant chemotherapy as compared to conventional breast surgery. We investigated whether oncoplastic breast surgery causes a delay in the onset of adjuvant chemotherapy in comparison to lumpectomy and mastectomy.
Material and methods: The study is a population-based cohort study. Within the nationwide registry of the Danish Breast Cancer Group (DBCG), we identified 1798 patients who received adjuvant chemotherapy following mastectomy, lumpectomy or oncoplastic breast surgery for early and unilateral invasive breast cancer. Women treated with neoadjuvant chemotherapy were excluded.
Results: We found no significant difference between the three groups (mastectomy, lumpectomy, oncoplastic breast surgery) in the time from biopsy to surgery (mean time 17.9, 17.0 and 18.3 days, respectively), the time from surgery to onset of adjuvant chemotherapy, nor total time from biopsy to the onset of adjuvant chemotherapy (mean time 52.7, 51.9 and 53.2 days, respectively).
Conclusions: Our study shows that oncoplastic breast surgery does not delay the onset of adjuvant chemotherapy in comparison with mastectomy and lumpectomy. Accordingly, patients should not be excluded from treatment with oncoplastic breast surgery due to concerns of delay in adjuvant chemotherapy.
Background
Adjuvant chemotherapy significantly reduces the recurrence and mortality rate of breast cancer [Citation1]. A detrimental impact on survival has been shown as a result of a major delay of adjuvant cancer treatment [Citation2]. Considerable effort has been put into accelerating the cancer treatment in Denmark. In 2009, an upper limit for the maximal duration of cancer diagnosis and initiation of cancer treatment was set at 18 working days by the Danish Health and Medicines Authority [Citation3].
As breast cancer has become a disease where the majority of patients can expect to be long-term survivors [Citation4], the demand for a cosmetically acceptable result after breast cancer surgery has increased. Oncoplastic breast surgery (OBS) aims at improving the esthetic outcome after breast cancer surgery and consists of two parts: removal of the tumor (lumpectomy) and a corrective plastic surgical procedure in order to restore the shape of the breast [Citation5].
Corrective procedures in OBS rely on techniques adopted from reduction mammoplasty, local flaps of breast parenchyma, fasciocutaneous flaps or perforator flaps. In Denmark, neither pedicle flaps (e.g., latissimus dorsi flap, transverse rectus abdominus musculocutaneous flap) nor free tissue transfer are used in OBS [Citation6]. In OBS, there are different challenges that may extend the time interval from the diagnostic biopsy to the onset of adjuvant chemotherapy compared with lumpectomy or mastectomy. Since both breast surgeons and plastic surgeons are involved in OBS, the more complicated logistic planning of the surgery could lead to postponed surgery. In addition, patients treated with OBS are subject to an extended surgical intervention due to the corrective procedures, which could increase the risk of surgical complications in terms of infection, hematoma, seroma and skin or flap necrosis. In many cases, a contralateral mammaplasty is conducted in conjunction with OBS which, in itself, may lead to complications.
Consequently, there are concerns that OBS could potentially be associated with the postponed initiation of adjuvant chemotherapy, which may lead to an inferior prognosis. The aim of this study was to investigate whether OBS causes a delay in the onset of adjuvant chemotherapy in comparison to traditional breast cancer surgery. The study is population based and benefits from the use of a Danish national database managed by the Danish Breast Cancer Group (DBCG) [Citation7], which prospectively collects detailed clinical data, histopathological status, information on treatment, and data on recurrence and mortality [Citation8]. Furthermore, treatment principles are standardized in Denmark according to European guidelines [Citation9] and national protocols designed by the DBCG [Citation8].
Material and methods
Population
In Denmark (2009–2013), there are ∼4900 new breast cancer cases annually [Citation10] of which around 70 and 30% are treated with lumpectomy and mastectomy, respectively [Citation11]. In the present study, patients were identified from the DBCG database [Citation8]. Inclusion criteria were: Women treated for primary operable breast cancer with mastectomy, lumpectomy or OBS in 2011 and 2012 and receiving adjuvant chemotherapy. Exclusion criteria were: Patients treated with neoadjuvant chemotherapy. In total, 1902 patients were identified. Subsequently, we excluded 39 patients with post-mastectomy breast reconstruction, 32 patients treated with mastectomy secondary to lumpectomy or OBS due to insufficient resection margins, 28 patients with incomplete data of onset of adjuvant chemotherapy and five patients with a negative time interval from surgery to onset of chemotherapy, due to incorrect registration. In total, 1798 patients were eligible for further analysis (95% of initial cohort).
Treatment
The surgical and adjuvant treatments were standardized according to DBCG guidelines; surgical treatment consisted of mastectomy, lumpectomy or OBS in combination with either Sentinel Lymph Node Biopsy (SLNB) or Axillary Lymph Node Dissection (ALND). Patients are offered ALND if metastasis is found in diagnostic biopsies from the axil (preoperatively ultrasonic examination) or in perioperative SLNB. Adjuvant chemotherapy was offered to patients according to risk profile. Patients allocated to adjuvant chemotherapy received three cycles of 3-weekly epirubicin 90 mg/m2 and cyclophosphamide 600 mg/m2 followed by three cycles of 3-weekly docetaxel 100 mg/m2 [Citation8]. Patients with hormone receptor positive tumor were also recommended endocrine treatment. Radiotherapy to the residual breast is mandatory following lumpectomy and thereby also in all cases of OBS, and the chest wall following mastectomy in patients younger than 70 if node positive or a tumor larger than 5 cm in diameter, and to regional lymph nodes in patients with macrometastasis.
Variables
The outcome of interest was time to initiation of adjuvant chemotherapy in three different treatment groups (mastectomy, lumpectomy and OBS). Day 0 was set on the date of the diagnostic biopsy. Time was measured from day 0 to onset of adjuvant chemotherapy. The time interval was separated in two periods from day 0 to day of surgery and from day of surgery to day of first series of adjuvant chemotherapy. In order to describe our patient population, we evaluated differences in age at the day of biopsy [years], BMI [kg/m2], tumor size [mm] measured preoperatively with ultrasonic imaging, type of axillary surgery, nodal status after histologic examination and contralateral corrective surgery.
Statistics
Differences in patient and tumor characteristics between groups were tested using One-way ANOVA. The time intervals from biopsy to surgery, surgery to adjuvant chemotherapy and total time from biopsy to adjuvant chemotherapy were not normally distributed (Kolmogorov–Smirnov test, p < .01) and non-parametric statistics (Wilcoxon’s Rank Sum Tests and Kruskal–Wallis Test) were used to test for differences. The level of significance was set to 5%. All statistical analyses were performed using SAS 9.1 (SAS Institute, Cary, NC).
Results
Out of the 1798 patients included in the study, 529 (29%) patients were treated by mastectomy, 824 (46%) patients were treated by lumpectomy and 445 (25%) patients were treated by OBS. Patient and disease characteristics are shown in . We found significant differences between the three groups when evaluating patient and disease characteristics (age, BMI, tumor size, insufficient resection margins, nodal status, type of axillary surgery and contralateral corrective surgery). Patients treated with mastectomy had a larger tumor size, and were more often node positive and treated by ALND than patients in the other groups. More patients treated with OBS also received contralateral corrective surgery (either mastopexy or reduction mammaplasty). Time from biopsy to surgery, surgery to onset of chemotherapy and biopsy to onset of chemotherapy are depicted in . The maximum time difference from biopsy to surgery was 1.3 days between the three groups (). The maximum time difference from surgery to onset of adjuvant chemotherapy was less than one day (0.7 day) between the three groups. There was no significant difference between the three groups (mastectomy, lumpectomy and OBS) in either time from biopsy to surgery (p = .256) or time from surgery to initiation of adjuvant chemotherapy (p = .054). The maximum time difference from biopsy to onset of adjuvant chemotherapy varied was 1.3 days, without significant difference between the groups (p = .833).
Figure 1. Presented in this figure are 1798 patients treated with mastectomy, lumpectomy and oncoplastic breast surgery in 2011 and 2012. The figure show 1., 2. and 3. quartile (box) and minimum and maximum values (whiskers).
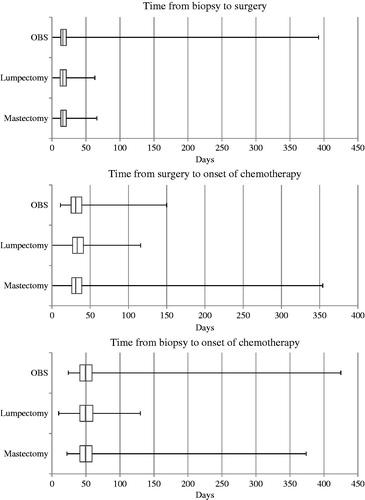
Table 1. Patient and disease characteristics in 1798 patients treated with surgery and adjuvant chemotherapy for breast cancer in 2011 and 2012.
Table 2. Time interval from diagnostic biopsy to surgery and from surgery to adjuvant chemotherapy among 1798 Danish women treated for operable breast cancer in 2011 and 2012.
Discussion
We found that OBS was not associated with a significant delay in the time interval from biopsy to surgery or in the time interval from surgery to adjuvant chemotherapy compared to patients undergoing lumpectomy or mastectomy. As shown in , some patients present with a very long delay. In total, 27 patients (1.5%) exceed 100 days, of whom two patients (0.1%) exceed 200 days, from biopsy to onset of adjuvant chemotherapy. The reason for the long delay is unknown.
The most common factors that could influence the time from surgery to initiation of adjuvant chemotherapy are postoperative complications in terms of hematoma, infection, tissue necrosis and wound rupture. As OBS did not postpone the initiation of adjuvant chemotherapy, the conditions mentioned are expected to be equally frequent among mastectomy, lumpectomy and OBS.
To the best of our knowledge, this is the largest study on the subject. The data was retrieved from the national DBCG register. The Danish population is broadly ethnically homogenous, well educated, and benefits from a uniform healthcare system with equal access to examination and treatment with full reimbursement. However, the external validity of this study is limited to populations with similar demographics, healthcare systems and treatment protocols. Selection bias is a potential limitation in our study and patients offered OBS might have superior general health. The higher BMI among women treated with lumpectomy may reflect selection bias, as lumpectomy requires a larger breast volume relative to the tumor size than mastectomy and OBS.
Due to the large sample size, we found statistically significant differences in age and BMI between groups. Nonetheless, mean age only varied by two years and mean BMI only varied between 25.1 and 26.4. This small difference was not considered clinically significant. As expected, patients treated by mastectomy had a significantly larger tumor size and were more often node positive compared to the other groups. As a result, significantly more patients treated by mastectomy underwent ALND. This more extensive axillary surgery could potentially increase the risk of postoperative complications and delay the time to adjuvant chemotherapy. However, still no significantly different delay was seen between groups.
Only two minor studies have previously examined the association between OBS and onset of adjuvant chemotherapy. Dogan et al. showed that chemotherapy was not delayed in 78 patients treated with OBS compared with mastectomy (n = 155 patients) and lumpectomy (n = 47 patients) [Citation12]. Equally, Kahn et al. showed no delay in chemotherapy in 31 patients treated with OBS in comparison with 138 patients treated with lumpectomy, mastectomy or mastectomy and immediate breast reconstruction [Citation13]. We confirm these findings of no difference in time from OBS to initiation of adjuvant chemotherapy in a large population-based study.
In addition, the logistic challenges in planning OBS with surgeons from different departments could potentially lead to a delay of the surgical treatment. If so, the time interval from biopsy to surgery would have been extended. No earlier studies have examined whether planning of interdisciplinary OBS affects the time interval from diagnostic biopsy to surgery. In this study, we found no difference in time from diagnosis to surgery between patients treated with OBS, lumpectomy or mastectomy. In Denmark, OBS is conducted in cooperation between breast surgeons and plastic surgeons. The finding in our study is dependent on close cooperation between breast surgeons and plastic surgeons.
Cold et al. [Citation14] have shown that the overall survival rate is unaffected when adjuvant chemotherapy is started within 3–13 weeks after definitive surgery. This finding was confirmed by Lohrisch et al. [Citation2]. Gagliato et al. found that time to adjuvant chemotherapy exceeding 61 days (corresponding to >9 weeks) decreased survival outcomes, in particular in patients with more advanced breast cancers [Citation15]. In our study, the time interval from surgery to onset of adjuvant chemotherapy was ∼34 days (34.2–34.9 days corresponding to ∼5 weeks) for the group treated with OBS. In all three groups, adjuvant chemotherapy appears to be initiated with a safe time margin to the documented critical levels of delay (9 and 13 weeks, respectively). Therefore, it is expected that OBS is not associated with an aggravated prognosis due to delay of onset of adjuvant chemotherapy.
Conclusions
Our study shows that OBS was not associated with delay from diagnostic biopsy to surgery or from surgery to initiation of adjuvant chemotherapy compared to mastectomy and lumpectomy. Accordingly, patients should not be excluded from treatment by OBS due to concerns of treatment delay.
Disclosure statement
The authors declare that they have no conflict of interest.
Additional information
Funding
References
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717.
- Lohrisch C, Paltiel C, Gelmon K, et al. Impact on survival of time from definitive surgery to initiation of adjuvant chemotherapy for early-stage breast cancer. J Clin Oncol. 2006;24:4888–4894.
- Danish Health and Medicines Authority. Pakkeforløb for brystkræft [Internet]. 2012 Jun [cited 2016 Aug 24]. Available from: http://www.sst.dk/∼/media/E43A4D438F814CD095A88C5A092BFA41.ashx
- Mouridsen HT, Bjerre KD, Christiansen P, et al. Improvement of prognosis in breast cancer in Denmark 1977–2006, based on the nationwide reporting to the DBCG Registry. Acta Oncol. 2008;47:525–536.
- Berry MG, Fitoussi AD, Curnier A, et al. Oncoplastic breast surgery: a review and systematic approach. J Plast Reconstr Aesthet Surg. 2010;63:1233–1243.
- Danish Breast Cancer Group (DBCG). Guideline for surgical treatment in Danish [Internet]. 2014 Apr [cited 2017 Jan 4]. Available from: http://dbcg.dk/PDF%20Filer/Kap_4_Kirurgisk_behandling_03.04.13.pdf
- Blichert-Toft M, Christiansen P, Mouridsen HT. Danish Breast Cancer Cooperative Group–DBCG: History, organization, and status of scientific achievements at 30-year anniversary. Acta Oncol. 2008;47:497–505.
- Moller S, Jensen MB, Ejlertsen B, et al. The clinical database and the treatment guidelines of the Danish Breast Cancer Cooperative Group (DBCG); its 30-years experience and future promise. Acta Oncol. 2008;47:506–524.
- Goldhirsch A, Wood WC, Gelber RD, et al. Meeting highlights: updated international expert consensus on the primary therapy of early breast cancer. J Clin Oncol. 2003;21:3357–3365.
- IARC. NORDCAN [Internet]. 2016 Aug [cited 2016 Aug 15]. Available from: http://www-dep.iarc.fr/NORDCAN/english/StatsFact.asp?cancer =180&country =208
- Christiansen P, Vejborg I, Kroman N, et al. Position paper: breast cancer screening, diagnosis, and treatment in Denmark. Acta Oncol. 2014;53:433–444.
- Dogan L, Gulcelik MA, Karaman N, et al. Oncoplastic surgery in surgical treatment of breast cancer: is the timing of adjuvant treatment affected?. Clin Breast Cancer. 2013;13:202–205.
- Kahn J, Barrett S, Forte C, et al. Oncoplastic breast conservation does not lead to a delay in the commencement of adjuvant chemotherapy in breast cancer patients. Eur J Surg Oncol. 2013;39:887–891.
- Cold S, During M, Ewertz M, et al. Does timing of adjuvant chemotherapy influence the prognosis after early breast cancer? Results of the Danish Breast Cancer Cooperative Group (DBCG). Br J Cancer. 2005;93:627–632.
- de Melo GD, Gonzalez-Angulo AM, Lei X, et al. Clinical impact of delaying initiation of adjuvant chemotherapy in patients with breast cancer. J Clin Oncol. 2014;1032:735–744.
