Abstract
Background: The early diagnosis and right treatment strategy of localized prostate cancer (PCa) remains problematic. In order to characterize the survival of PCa patients, we compared patients’ all-cause and cancer-specific mortalities between pre- and post-PSA periods by stage in Finland.
Material and methods: All PCa cases diagnosed in Finland between 1985 and 2013 (N = 91,329) were identified from the Finnish Cancer Registry (FCR). PCa stage at diagnosis was defined as localized, local node positive or metastasized. Standardized mortality ratios (SMRs), and relative and cause-specific survival were assessed by stage and introduction of PSA testing. The main limitation was the high proportion of men with unknown stage (28%).
Results: A clear decreasing trend in the SMR of PCa patients was evident when pre- and post-PSA eras were compared: for localized PCa, the SMR was 1.43 (95%CI 1.38–1.48) in 1985–1989 and 0.98 (95%CI 0.95–1.01) in 2000–2004, and for metastasized PCa, the SMRs were 4.51 (95%CI 4.30–4.72) and 3.01 (95%CI 2.89–3.12), respectively. Difference between cause-specific and relative survival was pronounced in localized PCa in post-PSA period: 10-year relative survival was 94.6% (95%CI 91.4–97.8) and cause-specific 84.2% (95%CI 82.9–85.5%). In metastasized PCa the difference was not that significant.
Conclusions: From 1985 to 2009, the SMR among men diagnosed with PCa decreased significantly in Finland. Among men with localized PCa, the SMR decreased even below that of the Finnish male population. This and the increased difference between relative and cause-specific survival reflects most likely selection of men to opportunistic PSA testing. The results highlight the importance of caution in the use of PSA testing in healthy men.
Introduction
Prostate cancer (PCa) in developed countries is currently characterized by high incidence and low mortality. PCa is usually diagnosed in old age, and disease progression is slow: in a Danish population-based study, the mean age of PCa diagnosis was 74.6 years [Citation1], and the 5-year age-standardized relative survival of PCa patients between 1999 and 2007 was approximately 90% in Finland and 85% in Scandinavia [Citation2]. A landmark Swedish study demonstrated the benign course/progression of well- or intermediately differentiated PCa at diagnosis even without initial treatment with curative intent [Citation3]. The PCa-specific 15-year mortality rate for men with low-risk PCa is low [Citation4,Citation5]. Among high-risk and metastasized PCa, mortality is dramatically different, and PCa poses a real risk of death [Citation6,Citation7].
Since the onset of PSA testing, the incidence of localized PCa has increased rapidly [Citation8] and in Finland, it seems to level-off after 2008 [Citation9]. The past increase led to a wide utilization of PCa treatments with curative intent, radical surgery in particular [Citation10]. Until recently, the curative treatments for localized PCa have been used liberally [Citation11]. However, now it is common to favor active surveillance and delay surgery or radiotherapy in localized Gleason 6 PCa [Citation12]. On the other hand, some PCa may have been undertreated, especially among the old age group ≥75 years for whom co-morbidities and life expectancy are major concerns [Citation13]. Among patients aged ≥75 years and with metastatic disease, androgen deprivation therapy has been the main treatment. Although this therapy provides palliation, little evidence about its efficacy in improving overall survival exists [Citation14]. The emphasis of PCa treatment is changing: a conservative approach with active surveillance is now common in localized low-risk PCa [Citation5], and the focus on radical treatment has moved toward advanced cases [Citation15].
The objective of this study was to assess the excess risk of death among men with various stages of PCa according to pre- and post-PSA periods. Furthermore, we estimated cause-specific survival to assess whether the decrease in excess risk is related to the selection of men due to opportunistic PSA testing.
Material and methods
Study population
The nationwide population-based Finnish Cancer Registry (FCR) collects information on incident cancers annually from hospitals, outpatient clinics and other healthcare facilities and separately from histopathological laboratories in Finland. Additionally, all deaths of cancer patients and death certificates where cancer is mentioned are reported to the registry by Statistics Finland. PCa cases diagnosed from 1985 until 31 December 2013 were retrieved from the FCR. In all, 91,329 PCa cases were identified. They were defined as localized, local node positive and metastasized at diagnosis based on the FCR data. Person-years were calculated from the date of PCa diagnosis to death, emigration or 31 December 2013, whichever occurred first. Age group (1 year) and calendar year-specific mortality rates for the Finnish male population were obtained from Statistics Finland (www.tilastokeskus.fi). In our study, years between 1985 and 1994 were defined as pre-PSA period and years after 1995 as post-PSA period.
The study protocol was approved by the IRB of the Southwestern Hospital District of Finland. The Finnish National Institute of Health and Welfare approved access to registry data (study number 182/5.05.00/2015).
Statistical analysis
The standardized mortality ratio (SMR) compares the mortality rates of PCa patients to those of the Finnish male population. The SMR is estimated as the ratio of observed and expected number of deaths standardized indirectly with age and calendar year. The observed number is the number deaths from any cause in the PCa cohort. The expected number for each standard variable stratum is derived by multiplying the population mortality rates by the person-years of the cohort [Citation16]. The patients were censored after 10 years of follow-up in order to make mortality of patients diagnosed in different calendar periods comparable. The 95% confidence intervals (CIs) were calculated assuming a Poisson distribution for observed deaths.
The relative survival ratio is defined as the ratio between the observed survival of the PCa patients and their expected survival. The expected survival was derived from the Finnish male population mortality rates stratified by age and calendar time by using the Ederer II method [Citation17]. The cause-specific survival with respect to deaths from PCa was estimated using a life table method in which the deaths due the other causes than PCa (or the deaths due the PCa when cause-specific survival is estimated with respect to other causes) are considered as censored events. Traditional direct age standardization was used to compare survival estimates between different groups. In each comparison, the age distribution of the patients diagnosed in the pre-PSA era (1985–1994) was used as the standard (four age groups: 0–59, 60–69, 70–79 and 80 years old and older).
Statistical analyses were conducted in statistical program R version 3.2.3 (R Development Core Team 2015) using popEpi package (http://cran.r-project.org/package=popEpi).
Results
Of all PCa patients (N = 91,329), about half had localized disease (N = 47,001, 51.5%) at diagnosis (). The stage was unknown in more than one-fourth of patients (N = 25,391, 27.8%). The number of local node positive cases was very low (N = 784, 0.9%) due to missing nodal status reports. The proportion of men with unknown cancer stage was higher in recent years ().
Figure 1. The numbers of newly diagnosed prostate cancer cases by calendar period and stage. All stages (black circles), localized disease (circles), metastasized (triangles), unknown (squares).
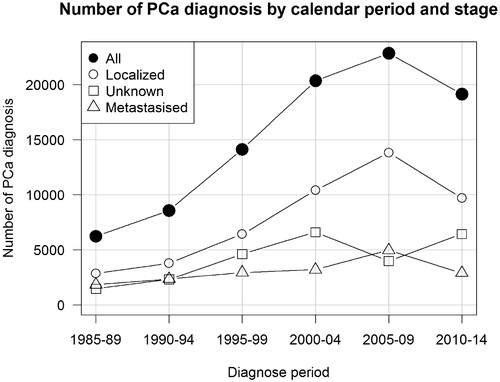
Table 1. Basic characteristics of the study population.
The number of all PCa diagnoses increased almost 4-fold from 1985–1989 (N = 6227) to 2005–2009 (N = 22,868) and mostly of localized stage. Most cases were of high age at diagnosis; 55.7% were aged 70 years or more (). Majority of men with localized PCa died from other reasons (N = 11,228) than PCa (N = 4058) during the first 10 years of follow-up, while majority of men with metastatic disease died from PCa during the same period. During the first 10 years of follow-up, the absolute number of deaths in localized PCa has been essentially the same from the pre-PSA-testing era (1730) to post-PSA-testing era (1722). However, the number of deaths due to other causes has increased (from 2973 to 5320). Among metastasized PCa patients, the absolute number of deaths due to PCa did not chang significantly from pre-PSA period (3121) to post-PSA period (3455), but increased due to other causes of death (from 759 to 1542 deaths) ().
The declining trend in SMRs was seen for whole-study population: SMR 2.08 (95% CI 2.02–2.14) in 1985–1989 to 1.35 (95%CI 1.32–1.38) in 2000–2004. The SMR for the overall period 1985–2009 was 1.55 (95% CI 1.54–1.57) (). The SMR for localized PCa decreased significantly over time (p < .001) from 1.50 (95% CI 1.44–1.57) in 1985–1989 to 0.98 (95%CI 0.95–1.01) in 2000–2004. Since the early 2000s the SMR among men diagnosed with localized PCa was lower compared to the SMR in the Finnish male population. In metastatic PCa, a similar declining trend (p < .001) was seen: the SMR was 4.51 (95% CI 4.30–4.72) in 1985–1989 and 3.01 (95%CI 2.89–3.12) in 2000–2004 (). In the group of unknown stage, the SMR was also decreasing over the time similarly to all PCa patients ().
Table 2. SMR by stage and year of diagnosis. Periods 1985–1994 represent the pre-PSA era and periods 1995–2004 the post-PSA era. Follow-up is limited to 10 years.
In metastasized PCa, the estimates of relative and cause-specific survival were similar in both pre- and post-PSA era. In the post-PSA era, the 10-year relative survival was 34.7% and the cause-specific survival 34.1% ( and ). In the pre-PSA period, the respective numbers were 16.9% and 14.1%. Also in localized PCa, cases diagnosed in the pre-PSA era the 10-year relative and cause-specific survival estimates were similar: 66.1% and 63.6%, respectively. However, in the post-PSA era, the difference between the 10-year relative (94.6%) and cause-specific (84.2%) survival was 10.4 percentage points. Between pre- and post-PSA era, the cause-specific survival with respect to the other causes of death than PCa increased 12.7 percentage points in localized PCA but only 1.7 percent points in metastasized ( and ). The results on SMR and survival did not change substantially when extending follow-up time up to 15 years.
Figure 2. Relative survival (Relative) and cause-specific survival with respect to PCa (Cancer) and other causes than PCa (Other) of localized and metastasized prostate cancers in pre- (1985–1994) and post- (1995–2004) PSA-testing eras.
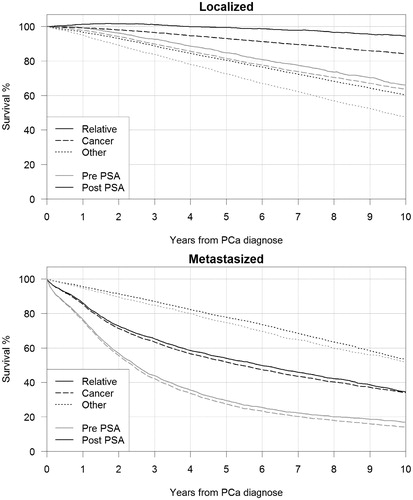
Table 3. Survival estimates for .
Discussion
In this nationwide population-based study on patients with diagnosed with PCa in Finland, we found that excess mortality of the patients compared to the national population and also cause-specific mortality due to PCa decreased considerably during the last three decades. The number of all PCa diagnoses increased dramatically over time, mostly due to increasing PSA testing since the mid-1990s and aging of the population.
The relative survival of men with localized PCa was clearly larger compared to the cause-specific survival in post-PSA era. This and their decreased other-cause mortality indicate that men diagnosed with localized PCa are on average healthier than the Finnish male population.
Our results are in line with a recent Swedish population-based study where men with low-risk PCa had lower all-cause mortality than corresponding PCa-free men [Citation18]. In the Swedish study, this conclusion was made due to lower mortality from cardiovascular diseases (CVD) in low-risk group. Our study showed declined all-cause mortality among men with localized PCa, but no division between high, intermediate and low risk was made. Increased trend in relative survival in this study was also observed in Europe during the last decade; the average 5-year relative survival of PCa patients steadily increased from 73.4% in 1999–2001 to 81.7% in 2005–2007 [Citation2].
Wide utilization of PSA testing typically results in overdiagnosis and causes lead-time bias in survival estimates. Lead time, the time by which PSA screening advances PCa diagnosis, has been reported by several studies, with mean lead times ranging from 3 to 12 years [Citation19]. Active use of PSA testing has doubled the incidence of PCa in developed countries [Citation20,Citation21]. Shen et al. reported that based on the findings from SEER database, PSA screening rate in the USA for men over 70 years was 71%, which has led to an additional diagnosis of 5.8 cases of early stage cancer and 3.9 cases receiving treatment for early cancer for every 1 less case of stage IV disease at initial diagnosis [Citation22]. According to a Swedish study that studied PSA-based screening, cumulated uptake of PSA testing in men aged 55–69 years in Sweden increased from zero in 1997 to 56% in 2007 [Citation23]. However, no studies concerning Finnish males and opportunistic screening have been reported. The impact of PSA screening on mortality in the Finnish arm of the European Randomized Study of Screening for Prostate Cancer (ERSPC) [Citation24] resulted in a non-significant result (HR 0.85, 95% CI 0.69–1.04). The European trial, however, reported no all-cause mortality reduction, but a significant reduction in PCa mortality [Citation25]. Still there is evidence that reduced PCa mortality over the last decades in high incidence countries, like Finland, is also explained by opportunistic PSA screening [Citation26,Citation27]. Contemporary PCa diagnostic strategy together with the improved life expectancy of men put substantial pressure on the disease’s economic burden from the healthcare system’s perspective [Citation28]. Recently, new, more effective but costly therapies for castration resistant PCa have been developed and approved for clinical use [Citation29]. Awareness of the disease’s impact on life expectancy at the individual and population levels thus is very important.
Our study was designed to cover the eras before and after the introduction of the PSA testing in Finland in the mid-1990. In localized PCa, favorable prognosis is likely to be due to the common use of PSA testing [Citation24] and active utilization of curative treatment. In addition to overdiagnosis and lead-time bias, opportunistic PSA testing has caused selection such that men with low risk of death from other causes (e.g., from the CVD) are over presented in localized PCa patients. Also in general, volunteers for prevention or screening trials tend to be healthier or more health conscious than the overall population; this has been denoted the ‘healthy volunteer effect’, and this effect is well described in the literature [Citation30]. It is presumable that usually these men seek preventive services and attend screening program. The test may also be more often available for men with higher socioeconomic status, for example, offered by occupational health services. This kind of selection can be seen from increased difference between relative and cause-specific survival estimates and from the mortality ratios below 1. Therefore, the SMRs and relative survival do not describe the excess mortality caused by the localized PCa itself and the trend in SMR reflects the effects of the selection and earlier diagnosis due to the PSA testing and advanced treatments.
The results show that there is no such a selection of healthier men with metastasized PCa as seen among patients with localized PCa. In metastasized PCa, over 10% increase in survival between pre- and post-PSA eras is mostly due new cancer treatments and the lead time [Citation19,Citation29]. Important factor contributing to the trend of improved survival is advanced cancer therapy, which has been utilized since 2004 [Citation31]. Furthermore, the metastatic (M1) population is heterogeneous, with the most convincing data on prognosis produced by the large SWOG8894 trial [Citation7]. This trial showed that also performance status and co-morbidities play an important role for prognosis.
This study’s strength is that it covers a national (close to 100%) cohort of all PCa cases, more than 90,000 patients, from a country with a high PCa survival. We demonstrate the PSA-based detection of PCa has an impact on survival and downgrade stage migration of PCa. In addition, we have complete 10-year follow-up information on all cases in both pre- and post-PSA era. FCR has extensive cause-of-death information for all cancer patients obtained from Statistics Finland. Similar population-based coverage of follow-up information is not available in many of the other European countries except for Nordic regions. Although randomized controlled studies provide the highest level of evidence, analyses of data from population-based registries yield important information for management of the effects of diagnosis and treatment practices in population level.
The main limitation of the study was the large proportion (27.8%) of men with unknown PCa stage. Another limitation is the rough division of the stage-groups as Gleason score and PSA value information was not available. Clinical information of the cancers is based on information sent by hospitals and pathological laboratories. Especially the clinical reports are often missing or with incomplete data. The lack of clinical information is unlikely to have affected the main conclusions. Owing to the scope of the investigation, the proportion of local node positive patients was small: Node positive stage was probably reported only if positive lymph nodes were found in pelvic lymphadenectomy specimens. Therefore, the SMR trend in this stage-group is inconclusive.
Conclusions
From 1985 to 2009, the SMR of men diagnosed with PCa decreased significantly in Finland. Among men with localized PCa the SMR decreased even below that of the Finnish male population. This and the increased difference between relative- and cause-specific survival reflects most likely opportunistic PSA testing among health conscious male population. This highlights the importance for better diagnostics tools for localized PCas.
Acknowledgements
We thank Dr. Karri Seppä for the very valuable discussions that substantially helped the authors to improve the interpretation of survival results.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Ingimarsdottir IJ, Rusch E, Engholm G, et al. Quality assessment of prostate cancer reports to the Danish Cancer Registry. Acta Oncol. 2016;55:24–29.
- de Angelis R, Sant M, Coleman MP, et al. Cancer survival in Europe 1999–2007 by country and age: results of EUROCARE-5 – a population-based study. Lancet Oncol. 2014;15:23–34.
- Johansson JE, Andren O, Andersson SO, et al. Natural history of early, localized prostate cancer. JAMA. 2004;291:2713–2719.
- Rider JR, Sandin F, Andren O, et al. Long-term outcomes among noncuratively treated men according to prostate cancer risk category in a nationwide, population-based study. Eur Urol. 2013;63:88–96.
- Klotz L, Vesprini D, Sethukavalan P, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol. 2015;33:272–277.
- Boccon-Gibod L, Bertaccini A, Bono AV, et al. Management of locally advanced prostate cancer: a European consensus. Int J Clin Pract. 2003;57:187–194.
- Glass TR, Tangen CM, Crawford ED, et al. Metastatic carcinoma of the prostate: identifying prognostic groups using recursive partitioning. J Urol. 2003;169:164–169.
- Neppl-Huber C, Zappa M, Coebergh JW, et al. Changes in incidence, survival and mortality of prostate cancer in Europe and the United States in the PSA era: additional diagnoses and avoided deaths. Ann Oncol. 2012;23:1325–1334.
- Finnish Cancer Registry, Cancer Statistics of Finland. [cited 2016 Nov 8]. Available from: http://tilastot.syoparekisteri.fi/?_inputs_&in.subset.area=%22-1L%22&in.subset.sex=%220L%22&in.subset.sites=%2232L%22&language=%22fi%22&submit=2&tabset_panel=%221%22&tabu=%221%22&value_theme=%22theme_inc%22&value_type=%22inc.rate_finland_2014%22.
- Lu-Yao GL, Potosky AL, Albertsen PC, et al. Follow-up prostate cancer treatments after radical prostatectomy: a population-based study. J Natl Cancer Inst. 1996;88:166–173.
- Ritch CR, Graves AJ, Keegan KA, et al. Increasing use of observation among men at low risk for prostate cancer mortality. J Urol. 2015;193:801–806.
- Loeb S, Folkvaljon Y, Makarov DV, et al. Five-year nationwide follow-up study of active surveillance for prostate cancer. Eur Urol. 2015;67:233–238.
- Houterman S, Janssen-Heijnen ML, Hendrikx AJ, et al. Impact of comorbidity on treatment and prognosis of prostate cancer patients: a population-based study. Crit Rev Oncol Hematol. 2006;58:60–67.
- Studer UE, Collette L, Whelan P, et al. Using PSA to guide timing of androgen deprivation in patients with T0-4 N0-2 M0 prostate cancer not suitable for local curative treatment (EORTC 30891). Eur Urol 2008;53:941–949.
- Touijer KA, Mazzola CR, Sjoberg DD, et al. Long-term outcomes of patients with lymph node metastasis treated with radical prostatectomy without adjuvant androgen-deprivation therapy. Eur Urol. 2014;65:20–25.
- Breslow NE, Day NE. Statistical methods in cancer research. Volume II – the design and analysis of cohort studies. IARC Sci Publ 1987;(82:):1–406.
- Seppa K, Hakulinen T, Laara E, Pitkaniemi J. Comparing net survival estimators of cancer patients. Stat Med 2016;35:1866–1879.
- van Hemelrijck M, Folkvaljon Y, Adolfsson J, et al. Causes of death in men with localized prostate cancer: a nationwide, population-based study. BJU Int 2016;117:507–514.
- Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101:374–383.
- Welch HG, Albertsen PC. Prostate cancer diagnosis and treatment after the introduction of prostate-specific antigen screening: 1986–2005. J Natl Cancer Inst. 2009;101:1325–1329.
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29.
- Shen X, Kumar P. Trade-off between treatment of early prostate cancer and incidence of advanced prostate cancer in the prostate screening era. J Urol. 2016;195:1397–1402.
- Jonsson H, Holmstrom B, Duffy SW, et al. Uptake of prostate-specific antigen testing for early prostate cancer detection in Sweden. Int J Cancer. 2011;129:1881–1888.
- Kilpelainen TP, Tammela TL, Malila N, et al. Prostate cancer mortality in the Finnish randomized screening trial. J Natl Cancer Inst. 2013;105:719–725.
- Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384:2027–2035.
- Stattin P, Carlsson S, Holmström B, et al. Prostate cancer mortality in areas with high and low prostate cancer incidence. J Natl Cancer Inst. 2014;106:dju007.
- Bray F, Lortet-Tieulent J, Ferlay J, et al. Prostate cancer incidence and mortality trends in 37 European countries: an overview. Eur J Cancer. 2010;46:3040–3052.
- Luengo-Fernandez R, Leal J, Gray A, et al. Economic burden of cancer across the European Union: a population-based cost analysis. Lancet Oncol. 2013;14:1165–1174.
- Crawford ED, Higano CS, Shore ND, et al. Treating patients with metastatic castration resistant prostate cancer: a comprehensive review of available therapies. J Urol. 2015;194:1537–1547.
- Pinsky PF, Miller A, Kramer BS, et al. Evidence of a healthy volunteer effect in the prostate, lung, colorectal, and ovarian cancer screening trial. Am J Epidemiol. 2007;165:874–881.
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512.
