Abstract
Background: The use of hypofractionated stereotactic body radiotherapy (SBRT) as primary treatment modality in clinically localized prostate cancer (PCa) is emerging, because the low α/β-ratio favors the use of high dose per fraction in PCa. There is a need for more data about SBRT, especially in high-risk PCa patients. The purpose of this retrospective study was to evaluate the safety and the short-term efficacy of robotic SBRT in a clinical patient cohort with localized PCa including also high-risk patients (D’Amico risk stratification).
Materials and methods: A total of 240 consecutive patients with clinically localized PCa were treated primarily with SBRT to total doses of 35 Gy or 36.25 Gy in 5 fractions using a robotic SBRT device (CyberKnife®). All risk groups (D’Amico risk stratification) were represented as follows: 48 (22%), 59 (27%) and 111 (51%) of the patients representing low-, intermediate- and high-risk group, respectively. Data on acute and intermediate-term toxicities and early PSA responses were analyzed.
Results: Neither acute grade 3 or higher GU nor rectal toxicity was observed. Regardless of the fact that 29 (13.3%) patients experienced intermediate-term toxicity requiring diagnostic interventions, the rates of intermediate-term grade 3 GU, rectal and infectious toxicity were low, 1.8%, 0.9% and 1.4%, respectively. A biochemical relapse was observed in ten (4.6%) patients. With the median follow-up time of 23 months the biochemical relapse-free survival (bRFS) rate was 100%, 96.6% and 92.8% in low-, intermediate- and high-risk group, respectively.
Conclusions: The toxicity of robotic SBRT in a large clinical cohort of PCa patients was tolerable and the early PSA response was good in all risk groups. The hypofractionated SBRT offers a possibility to high dose per fraction and to provide the whole radiotherapy treatment within two to three weeks.
Introduction
Prostate cancer (PCa) is the most common solid tumor among men worldwide with approximately one million new cases diagnosed annually [Citation1]. There are several treatment options for localized PCa, yet no consensus regarding the optimum primary treatment exists [Citation2]. For low and intermediate-risk PCa the prognosis is good irrespective of which initial treatment modality is chosen – i.e., either radical prostatectomy or radiotherapy (RT) in its different forms – [Citation3], and thus the rate and intensity of toxicity are important factors in the treatment selection.
For decades, the majority of PCa patients have been treated with external beam radiotherapy (EBRT) using conventional fractionation (2 Gy fractions given five times a week to a total dose of 66–76 Gy). Total dose escalation up to 80 Gy has been shown to achieve a better outcome in terms of reduced biochemical relapses [Citation4]. Several studies have shown PCa to have a low α/β-ratio (∼1.5 Gy) in comparison to most solid tumors (α/β-ratio ∼10 Gy). In a study suggesting a low α/β-ratio in PCa (1.5 Gy), Brenner et al. analyzed two mature data sets, one using conventional EBRT and the other using low dose-rate brachytherapy [Citation5]. Subsequent experimental and clinical studies have suggested α/β-ratio for PCa ranging from 1.0 to 1.7 Gy in all three risk groups [Citation6,Citation7]. A large review including over 14,000 PCa patients in 25 different studies reported similar results [Citation8].
Assuming the α/β-ratio of PCa truly being this low, delivering fewer fractions with higher doses per fraction delivered in a shorter overall treatment time, i.e., hypofractionation, would be advantageous especially in terms of radiobiological efficacy. Hypofractionation in PCa RT should also reduce the probability of late toxicities in the surrounding healthy tissues with the α/β-ratio ∼3 Gy. In addition, extreme hypofractionation in five treatment sessions in comparison to daily visits over seven to eight weeks could improve both the patient’s motivation and his quality of life.
The use of SBRT as the primary treatment modality in clinically localized PCa is emerging, and there are already reports of hypofractionated SBRT, delivered either by a robotic image guided device or by a gantry based SBRT device, in the treatment of low- and intermediate-risk PCa [Citation9–13]. However, there is a need for more data of SBRT especially in high-risk PCa patients. The purpose of this retrospective study was to evaluate the safety and the short-term efficacy of robotic SBRT in localized PCa. The primary objective of the study was to analyze and evaluate the acute and intermediate-term toxicities and the initial PSA responses.
Materials and methods
The study population is a clinical cohort of 240 consecutive patients diagnosed with localized, biopsy proven PCa and treated between April 2012 and March 2015. This study includes the patients who were referred to radiotherapy by urologists following the patients’ decisions to request active radical treatment and not preferring surgery.
The radiotherapy was delivered by a robotic SBRT device (CyberKnife®, Accuray Inc., Sunnyvale, CA). The study was approved by the Ethics Committee of the North-Savo Health Care District.
Treatment planning and delivery
In order to safely deliver higher doses per fraction, the delivery of radiation to a moving target has to be precise. The optimal way to localize the target during the robotic image guided radiotherapy, is to place four gold fiducials into the prostate under ultrasound guidance. A non-contrast pelvic computer tomography (CT) with 1 mm slice thickness and a pelvic 1.5T- or 3.0T-magnetic resonance images (MRI) were obtained 7–10 days after the fiducial placement. The MRIs were fused with the CT images to precisely delineate the target volume as well as the organs at risk. A few patients were unable to undergo the MRI either due to physical limitations (severe obesity) or due to a cardiac pacemaker. All patients were advised to undergo bowel prep with bisacodyl before the planning imaging and each treatment session.
The prostate and the proximal seminal vesicles were considered as the clinical target volume (CTV). In order to achieve the planning target volume (PTV) for low- and intermediate-risk patients, CTV was expanded by 3–5 mm throughout, except for posteriorly always 3 mm to protect the anterior wall of the rectum. For high-risk patients, CTV was expanded by 5mm throughout, except for 3 mm posteriorly. The delineated organs at risk (OAR) were rectum, urethra, bladder, bladder wall, femoral heads, penile bulb and testes. The prescribed total dose was 35 or 36.25 Gy in 5 fractions of 7 or 7.25 Gy, respectively, delivered on every other day by CyberKnife®. The dose was normalized on average to the 85% (range, 76–90%) isodose line. The dose targets and constraints used for treatment planning are presented in . During a typical 30–45 min treatment session, the fiducials were tracked at 15–60 s intervals by orthogonal X-ray imaging and corresponding adjustments were automatically made by the robotic treatment device.
Table 1. Dose–volume constraints for treatment planning.
Follow-up and toxicity assessment
After SBRT, the patients were followed-up with individual control schemes. All relevant patient records were systematically reviewed according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.03 to assess grade 3 or higher genitourinary (GU) and rectal toxicities. Acute toxicity was defined as an adverse event presenting within the first 3 months after treatment, and intermediate-term toxicity presenting later during the current median follow-up of 23 months, up to the maximum of 46 months. A benign bounce was determined as a PSA rise of 0.2 ng/ml or above with a subsequent, spontaneous decline back to the previous nadir or lower. A biochemical relapse was defined according to the Phoenix definition, i.e., PSA nadir +2 ng/ml [Citation14].
Results
Patient demographics
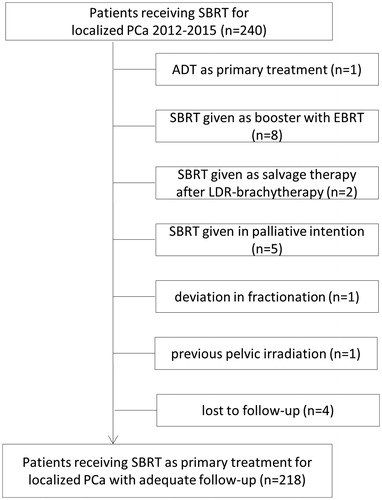
Twenty-two patients were excluded from the analysis due to the reasons shown in . Thus, 218 patients were treated with SBRT as their primary curative treatment and were included in this analysis. The median follow-up time was 23 months. The median age of the patients was 70 years (range, 47–86). All risk groups (D’Amico risk stratification) were represented as follows: 48 (22%), 59 (27%) and 111 (51%) of the patients represented low-, intermediate- and high-risk group, respectively. Ten (4.6%) of the high-risk patients presented with all of the high-risk characteristics.
Androgen deprivation therapy (ADT) was given to 14 (29.2%), 30 (50.8%) and 98 (88.3%) patients in the low-, intermediate- and high-risk groups, respectively. Previously, eight patients in the low-risk group had been in active surveillance for the median of 29 months (range, 16–83 months). Following the disease evolvement to intermediate or high-risk PCa, SBRT was delivered with combining ADT for ≤6 months. In the intermediate-risk group, the duration of ADT was most commonly 6–24 months, except in nine patients, who received ADT for over 24 months. In the high-risk group 47 (48%) patients used ADT for 24 months or longer. Detailed patient demographics are shown in .
Table 2. Patient demographics.
Dosimetric data
Initially, the dose level of 36.25 Gy was chosen [Citation12]. After the data on safety and sustained efficacy of SBRT in low- and low-intermediate risk (Gleason score being 3 + 4) PCa emerged [Citation15], a reduced dose level of 35 Gy was applied for these particular risk groups. Thirty-seven (17%) patients were treated with 35 Gy and 181 (83%) with 36.25 Gy in five fractions. The median duration of the treatment was 10 days (range, 8–21 days).
Toxicity
Genitourinary toxicity
Acute obstruction requiring daily catheterization was observed in three (1.4%) patients. No grade 3 or higher acute toxicity was observed.
Intermediate-term GU toxicities requiring diagnostic interventions, either cystoscopy or ultrasound examination of the urinary tract or both, were observed in 19 (8.7%) patients. Seventeen of them were treated to the total dose of 36.25 Gy, 16 received adjuvant ADT and five had experienced a transurethral resection (TURP) at 2, 4, 8, 51 and 100 months prior to SBRT. The most prevalent GU toxicities were urinary frequency (eight patients, 3.7%), hematuria (six patients, 2.8%), urinary leakage (four patients, 1.8%) and nocturia (three patients, 1.4%). In addition, two patients (0.9%) required daily catheterization. The median time to observed toxicity was 14 months (range, 9–38 months). Cystoscopy was performed for 15 (6.9%) patients, in which radiation reaction was observed in eight (3.7%) and urethral strictures in two (0.9%) patients. An ultrasound examination of the urinary tract, all with normal findings, was performed on six (2.8%) patients.
The rate of intermediate-term grade 3 GU toxicity was 1.8%. An elective operative intervention, either TURP or incision of the bladder neck, was indicated for four (1.8%) patients for symptom relief. However, the three patients with strictures who were offered a TURP, refused. No intermediate-term grade 4 GU toxicity was observed.
Rectal toxicity
Acute rectal toxicity significantly influencing daily quality of life, i.e., bleeding from the rectum starting five days after SBRT and detected in proctoscopy, was observed in one (0.4%) patient. Administration of a hydrocortisone enema resolved the symptom with no later toxicity. None of the patients had acute grade 3 rectal toxicity.
Intermediate-term rectal toxicity requiring diagnostic interventions, either a colonoscopy or a sigmoidoscopy, was observed in seven (3.2%) patients. All of these patients were treated to the total dose of 36.25 Gy and four received adjuvant ADT. The most prevalent rectal toxicity was bleeding (five patients, 2.3%). In addition, excessively mucous stools and frequency in bowel movements were observed, each in one (0.4%) patient. The median time to the observed toxicity was 13 months (range, 6–17 months). Radiation proctitis was observed in four (1.8%) and internal hemorrhoids in two (0.9%) patients.
In order to provide symptom relief, cauterization was indicated and performed on two (0.9%) patients experiencing intermediate-term grade 3 rectal toxicity. No intermediate-term grade 4 rectal toxicity was observed.
Infectious toxicity
Seven (3.2%) patients experienced prolonged infectious problems, i.e., urinary tract infections (UTI), epididymitis or prostatitis. Six of these patients were treated to the total dose of 36.25 Gy and four received ADT. Their symptoms started usually soon after the treatment and were either prolonged or intermittently recurrent for months. Either an ultrasound examination of the urinary tract, cystoscopy or both, was performed in three (1.4%) patients, all with normal findings in the ultrasound but irritation of the bladder neck in cystoscopy. In order to provide symptom relief, per oral antibiotics, alpha-adrenergic blocking agents, anti-inflammatory analgesics and tadalafil were used. Two patients with recurrent UTI were successfully treated with repeated chondroitin sulfate solution intravesical instillations.
In order to provide symptom relief, three (1.4%) patients needed multiple treatments and elective interventions; including intravenous medication in two, and hospitalization, circumcision and elective dorsal incision, each in one patient. All three cases represent CTCAE v4.03 grade 3 infectious toxicity. No grade 4 infectious toxicity was observed.
Early PSA response
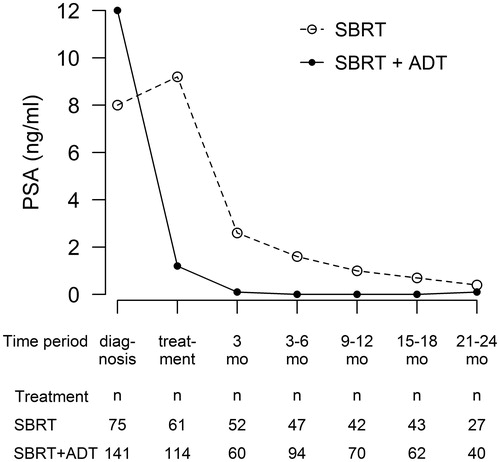
The median PSA follow-up time was 23 months (range, 1–46 months). The median PSA at the time of diagnosis was 10.0 ng/ml (range, 1.3–300.0 ng/ml) and it declined from the pretreatment median of 2.8 ng/ml (range, 0–30.6 ng/ml) to 0.2 ng/ml (range, 0–3.3 ng/ml) at 9–12 months post treatment.
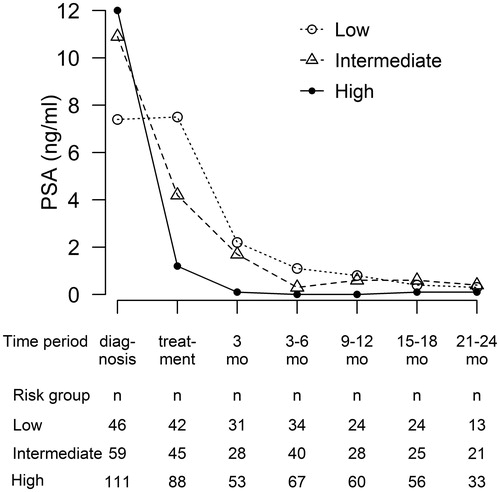
Seventy-six (35%) patients were treated with SBRT monotherapy: in them, the PSA declined steadily from the median pretreatment value of 9.2 ng/ml (range, 2.1–30.6 ng/ml) to the median of 1.0 ng/ml (range, 0.1–3.3 ng/ml) at 9–12 months post treatment. For the 142 (65%) patients, who received also ADT, the median PSA at the time of diagnosis was 12.0 ng/ml (range, 1.5–300.0 ng/ml) and the median pretreatment PSA was 1.2 ng/ml (range, 0.1–29.0 ng/ml). The PSA nadir (median 0.04 ng/ml, range 0–3.7 ng/ml) was reached already at 3–6 months after SBRT. PSA responses in different treatment and risk groups are presented in and .
A biochemical relapse was recorded in ten (4.6%) patients, of whom eight had a high-risk and two had an intermediate-risk disease. The median time to the biochemical relapse was 28 months (range, 11–46 months). The biochemical relapse-free survival rates (bRFS) at 23 months were 100%, 96.6% and 92.8% in the low-, intermediate- and high-risk groups, respectively.
Twenty-two (10.1%) patients experienced a benign PSA bounce with a median of 0.5 ng/ml (range, 0.2–1.7 ng/ml) and the median time to the first PSA bounce was 12 months (range, 6–27 months). At the time of the cutoff date, six patients had died; one due to another malignancy (adenocarcinoma of the gastro-oesophageal junction) and five due to other severe co-morbidities. As expected with a median of 23 months of follow-up, no deaths occurred which were attributable to PCa.
Discussion
Toxicity
The present study reports the first Nordic experience of robotic SBRT as the primary treatment modality in clinically localized PCa with or without ADT. Our final analysis of non-selected clinical cohort includes 218 PCa patients, half of them with high-risk disease. To the best of our knowledge, this is one of the largest European cohorts in this setting.
The study reveals that robotic SBRT was well tolerated in this clinical cohort of patients. Acute toxicity was mild, and neither acute grade 3 or higher GU nor rectal toxicities was observed. The rate of acute toxicity is in line with earlier reports in which acute grade 3 GU toxicity defined either by CTCAE or the Radiation Therapy Oncology Group (RTOG) scale has varied between 0–6.3% and acute grade 3 rectal toxicity is seen only rarely [Citation10,Citation11,Citation16,Citation17]. The percentage of cT3-4 tumors in our cohort was 32.1%, implicating locally more advanced tumors, and 29 (13.3%) patients experienced intermediate-term toxicity requiring diagnostic interventions. Still, the rates of intermediate-term grade 3 GU, rectal and infectious toxicity were low, 1.8%, 0.9% and 1.4%, respectively. All patients experiencing grade 3 toxicity had been treated to the higher dose of 36.25 Gy, which highlights the need of further dosimetric analysis of the PCa patients treated with SBRT.
Prolonged infectious toxicity has not been reported earlier. One explanation for the infectious toxicity could be that our cohort was a genuine clinical patient population, possibly including patients with significant urinary symptoms i.e., symptoms that would be considered as exclusion criteria in PCa SBRT trials [Citation16].
In the previously published data, the rates of both late grade 3 GU and rectal toxicity have varied between 0 and 5% whereas no evidence of grade 4 toxicity has been reported [Citation10–12,Citation15,Citation18]. The pooled analysis of the early and late toxicity data of the 1100 SBRT treated localized PCa patients may in future provide a larger perspective to the rate of and grading of GU and rectal toxicity [Citation19] as late toxicity can evolve even several years after SBRT [Citation13].
PSA response
During our median follow-up time of 23 months, a biochemical relapse was observed in ten (4.6%) patients. The bRFS for low and intermediate-risk groups were 100% and 98.3%, respectively, which are in line with previously published data [Citation9–11,Citation16]. There is evidence of durable disease control after SBRT among these risk groups with median follow-up times extending to 5–7 years [Citation15,Citation18,Citation20]; thus SBRT is a considerable treatment option for low- and intermediate-risk localized PCa in centers with the appropriate equipment and sufficient expertise.
Generally, patients with a localized high-risk PCa have a poorer prognosis compared to patients with low- or intermediate-risk disease [Citation21]. Their radical treatment options include prostatectomy (open, laparoscopic or robot-assisted), EBRT with adjuvant ADT and EBRT alone yielding a 10 year cancer specific survival of 88–92% [Citation22]. EBRT with high dose-rate brachytherapy boost to prostate is also one treatment option yielding a seven year bRFS of 86% [Citation23].
In this study, which had a median follow-up time of 23 months, among the 111 high-risk patients the bRFS was 93.8%. The majority (88.5%) of the high-risk patients also were administered ADT, which may partly explain the excellent results. There are only limited published data on the efficacy of SBRT with or without ADT in this risk group [Citation11,Citation15,Citation16,Citation19,Citation24]. In the largest pooled analysis including 125 high-risk PCa patients, the 5 year bRFS was 81% [Citation19]. In another study of 97 high-risk patients treated either with SBRT or SBRT + EBRT to the pelvic area, the 5 year biochemical disease-free survival was 68%. The pelvic EBRT did not improve the results [Citation24].
The use of hypofractionated SBRT as the primary treatment modality in localized high-risk PCa is emerging. Previous clinical results support the hypothesis of low α/β ratio of about 1.4 Gy in all three risk groups in PCa [Citation7]. In addition, it has been shown that prolonging the treatment duration in EBRT can exert a detrimental effect on disease-free survival, thus favoring a shorter total time for the treatment course [Citation25]. The abovementioned radiobiological factors were the main motives for treating also high-risk PCa patients with SBRT in our cancer center. The patients were informed that no evidence-based results of the efficacy of this particular treatment modality in high-risk group existed as yet. Nonetheless, the patients opted for SBRT as their treatment modality over the intensity-modulated radiation therapy delivered with conventional 2 Gy per fraction mainly because of the long distances to RT providers and the significantly shorter treatment period (5 visits in 2 weeks compared to 38 visits in 7.5 weeks). In our institution, robotic SBRT has become the primary radiotherapeutic treatment modality for localized PCa in all D’Amico risk groups unless presenting with contraindications, such as a suspicion of pelvic lymph node involvement or a large prostate volume (> 100cc).
The short-term efficacy results of this study are encouraging in all risk groups, although a longer follow-up will be required to evaluate in greater detail the long-term disease outcomes. The obvious limitations of this study are its retrospective nature and the relatively short follow-up time. The latter limitation cannot be overcome but the former was handled by scrutinizing only the clinically relevant toxicity.
Conclusions
This study reports the toxicity and the short-term efficacy of robotic SBRT in a large clinical cohort of localized PCa patients. The toxicity was tolerable; neither acute grade 3 or higher GU nor rectal toxicity was observed, and the rates of intermediate-term grade 3 GU, rectal and infectious toxicity were low. In addition, no intermediate-term grade 4 toxicity was observed. All of the grade 3 toxicity was observed among the patients treated to the higher dose of 36.25 Gy which highlights the need of further dosimetric analysis of the PCa patients being treated with SBRT. The early PSA response results add to the evidence of the good disease control of low and intermediate-risk PCa treated with SBRT. Furthermore, the results are encouraging also in the high-risk patient group. Thus, we conclude that hypofractionated SBRT will most probably become one of the treatment options for localized PCa because the low α/β-ratio favors the use of high dose per fraction, and the high dose per fraction makes it possible to deliver the whole radiotherapy treatment within two to three weeks.
Acknowledgements
The authors acknowledge biostatistician Tuomas Selander for graphical expertise.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- WHO Cancer Registry. Prostate cancer estimated incidence, mortality and prevalence worldwide in 2012; 2012. Available from: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
- Parker C, Gillessen S, Heidenreich A, et al. Cancer of the prostate: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26:v69–v77.
- Mottet N, Bellmunt J, Briers E, et al. EAU ? ESTRO – SIOG Guidelines on Prostate Cancer 2016. Available from: http://uroweb.org/guideline/prostate-cancer/.
- Kalbasi A, Li J, Berman A, et al. Dose-escalated irradiation and overall survival in men with nonmetastatic prostate cancer. JAMA Oncol. 2015;1:897–906.
- Brenner DJ, Hall EJ. Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys. 1999;43:1095–1101.
- Proust-Lima C, Taylor JM, Sécher S, et al. Confirmation of a low α/β ratio for prostate cancer treated by external beam radiation therapy alone using a post-treatment repeated-measures model for PSA dynamics. Int J Radiat Oncol Biol Phys. 2011;79:195–201.
- Miralbell R, Roberts SA, Zubizarreta E, Hendry JH. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: α/β = 1.4 (0.9-2.2) Gy. Int J Radiat Oncol Biol Phys. 2012;82:e17–e24.
- Dasu A, Toma-Dasu I. Prostate alpha/beta revisited – an analysis of clinical results from 14 168 patients. Acta Oncol. 2012;51:963–974.
- Friedland JL, Freeman DE, Masterson-McGary ME, et al. Stereotactic body radiotherapy: an emerging treatment approach for localized prostate cancer. Technol Cancer Res Treat. 2009;8:387–392.
- McBride SM, Wong DS, Dombrowski JJ, et al. Hypofractionated stereotactic body radiotherapy in low-risk prostate adenocarcinoma: preliminary results of a multi-institutional phase 1 feasibility trial. Cancer. 2012;118:3681–3690.
- Chen LN, Suy S, Uhm S, et al. Stereotactic body radiation therapy (SBRT) for clinically localized prostate cancer: the Georgetown University experience. Radiat Oncol. 2013;8:58.
- Katz AJ, Santoro M, Ashley R, et al. Stereotactic body radiotherapy for organ-confined prostate cancer. BMC Urol. 2010;10:1.
- Avkshtol V, Dong Y, Hayes SB, et al. A comparison of robotic arm versus gantry linear accelerator stereotactic body radiation therapy for prostate cancer. Res Rep Urol. 2016;8:145–158.
- Roach M, 3rd, Hanks G, Thames H Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO phoenix consensus conference. Int J Radiat Oncol Biol Phys. 2006;65:965–974.
- Katz AJ, Santoro M, Diblasio F, et al. Stereotactic body radiotherapy for localized prostate cancer: disease control and quality of life at 6 years. Radiat Oncol. 2013;8:118.
- Bolzicco G, Favretto MS, Satariano N, et al. A single-center study of 100 consecutive patients with localized prostate cancer treated with stereotactic body radiotherapy. BMC Urol. 2013;13:49.
- Detti B, Bonomo P, Masi L, et al. Cyberknife treatment for low and intermediate risk prostate cancer. Cancer Investig. 2015;33:188–192.
- Katz A, Kang J. Stereotactic body radiotherapy as treatment for organ confined low- and intermediate-risk prostate carcinoma, a 7-year study. Front Oncol. 2014;4:240.
- King CR, Freeman D, Kaplan I, et al. Stereotactic body radiotherapy for localized prostate cancer: Pooled analysis from a multi-institutional consortium of prospective phase II trials. Radiother Oncol. 2013;109:217–221.
- King CR, Brooks JD, Gill H, et al. Long-term outcomes from a prospective trial of stereotactic body radiotherapy for low-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2012;82:877–882.
- D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974.
- Boorjian SA, Karnes RJ, Viterbo R, et al. Long-term survival after radical prostatectomy versus external-beam radiotherapy for patients with high-risk prostate cancer. Cancer. 2011;117:2883–2891.
- Hoskin PJ, Rojas AM, Bownes PJ, et al. Randomised trial of external beam radiotherapy alone or combined with high-dose-rate brachytherapy boost for localised prostate cancer. Radiother Oncol. 2012;103:217–222.
- Katz A, Kang J. Stereotactic body radiotherapy with or without external beam radiation as treatment for organ confined high-risk prostate carcinoma: a six year study. Radiat Oncol. 2014;9:1.
- D’Ambrosio DJ, Li T, Horwitz EM, et al. Does treatment duration affect outcome after radiotherapy for prostate cancer? Int J Radiat Oncol Biol Phys. 2008;72:1402–1407.