Introduction
In the past decade, definitive stereotactic body radiation therapy (SBRT) has become more prevalent in the management of localized prostate cancer, with available evidence suggesting comparable efficacy and toxicity profiles to conventionally fractionated external beam radiation therapy (EBRT) [Citation1]. Multiple trials have been published in support of moderate hypofractionation [Citation2–5], and the available SBRT literature is supportive of excellent oncologic outcomes with acceptable toxicity [Citation6,Citation7]. Furthermore, large-scale retrospective comparative effectiveness research has demonstrated a lower total treatment cost for SBRT as compared with conventionally fractionated intensity modulated radiation therapy (IMRT) [Citation8], which is the current standard of care for patients undergoing EBRT.
Typically acute urinary symptoms peak in the week following SBRT, with a return to baseline over the ensuing months [Citation9]. Alpha-adrenergic antagonists and low-dose steroids are frequently used to mitigate these symptoms, and relatively few patients will go on to suffer from clinically significant long-term morbidity. Previous studies have examined the effect of SBRT dosimetry and its effect on late urinary toxicity [Citation10–13], but the specific risk factors for acute urinary toxicity remain undefined. Given the intimate anatomic relationship between the prostate, urethra, and bladder, the role of radiation dose to other genitourinary organs-at-risk (OARs) has been extensively evaluated in patients undergoing brachytherapy and conventionally fractionated EBRT [Citation14]. Published studies suggest that dose to the bladder neck, or trigone, is an important predictor of urinary toxicity in patients receiving definitive radiotherapy [Citation15,Citation16]. To better understand the nature of acute urinary morbidity caused by prostate SBRT, we instituted a prospective institutional protocol to investigate and identify clinical and dosimetric predictors of acute urinary morbidity seven days following SBRT for clinically localized prostate cancer.
Methods
Patients eligible for study inclusion had localized prostate cancer who were deemed candidates for definitive SBRT. Androgen deprivation therapy (ADT) was not considered a contraindication to treatment with SBRT monotherapy. All patients were treated prophylactically with oral alpha-adrenergic antagonist medication (e.g. tamsulosin) stating 5 d prior to SBRT delivery unless medically contraindicated.
SBRT treatment planning was performed according to institutional protocol, and has been previously described [Citation17]. The clinical target volume (CTV) consisted of the entire prostate and proximal seminal vesicles. A 3 mm posterior expansion and 5 mm expansion in all other directions was used to generate a planning target volume (PTV). The bladder neck was contoured retrospectively for all patients by a single physician (MCR) and delineated anatomically from the bilateral cystoureteral interfaces to the internal urethral orifice. The prostatic urethra was typically contoured prospectively using MR guidance; in patients for whom the urethra could not be identified, its location was estimated as a 10 mm cylinder structure. The bladder wall was approximated as a 5 mm internal shell structure of the whole bladder contour. Inverse plans were generated with a prescription dose of 35–36.25 Gy in five fractions to the PTV. Treatment was delivered using the CyberKnife (Accuray Inc., Sunnyvale, CA, USA) robotic radiosurgical system. A short-term steroid taper consisting of dexamethasone over 14 days was employed for patients with significant urinary symptoms.
Patients completed an IPSS questionnaire on the day of the first SBRT fraction. They were contacted by phone 7 days following the final fraction of SBRT in order to conduct an additional IPSS questionnaire. Student’s t-test was used to assess significance of change in IPSS from baseline levels at 7 d, 1 month, and 3 months post-SBRT. Cumulative DVHs were extracted from the treatment planning software and converted to differential DVHs using custom software written in MATLAB (MathWorks Inc., Natick, MA, USA). Acute urinary symptom flare was defined as an increase in IPSS of 5 points or more with an absolute score of at least 15 points at seven days post treatment. Absolute and relative volume models of differential DVH data were utilized for each structure and analyzed for significant predictors of toxicity in 0.1 Gy increments using custom software written in MATLAB. Univariate logistic regression was performed using PTV, bladder, bladder wall, bladder neck, and prostatic urethra DVH data along with patient and disease related factors to identify significant predictors of acute urinary symptom flare. Factors meeting a specified cutoff of p < 0.1 on univariate analysis were included in a multivariable regression using feature selection with a significance threshold of p < 0.05.
Results
Between May 2013 and August 2014, 103 men were treated definitively for localized prostate cancer with robotic SBRT. Baseline patient and tumor characteristics can be found in the Supplementary material. At seven days following treatment, 23 patients (22.3%) experienced acute urinary symptom flare. On univariate analysis, patient age, baseline PSA, baseline IPSS, clinical T stage, pathologic Gleason score, D’Amico risk classification group, and ADT usage were not associated with acute urinary symptom flare. However, prostatic volume was a significant predictor of acute urinary symptom flare at 7 d post-treatment (OR 1.26, 95% CI 1.05–1.52, p = 0.012). Patients whose prostatic volume was less than the median experienced a 12% rate of acute urinary symptom flare, compared to 33% in patients whose prostatic volume was greater than 36 cc ().
Figure 1. Predictors of acute urinary flare. Dark bars indicate the incidence of acute urinary symptom flare in patients whose prostate volume, bladder wall D15.5%, or combination were below the median value for the entire cohort. Light bars indicate the incidence of acute urinary symptom flare in patients whose prostate volume, bladder wall D15.5%, or combination were above the median value for the entire cohort.
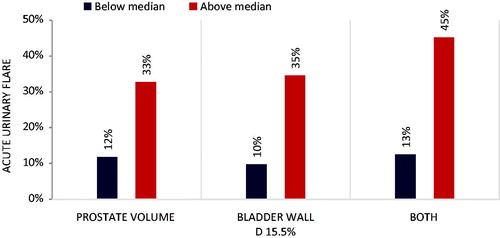
Univariate logistic regression identified predictors of urinary flare (p < 0.1) for incorporation into a multivariable regression model. The dose to the hottest 15.5% of the bladder wall (D15.5%) was identified as the most significant predictor of acute urinary symptom flare (OR 1.68, 95% CI 1.05–2.67, p = 0.029), as was the volume of the bladder wall receiving at least 14.2 Gy (V14.2 Gy, OR 1.04, 95%CI 1.00–1.09, p = 0.079). Patients whose D15.5% was less than the median value of 32.6 Gy experienced a 10% rate of acute urinary symptom flare, compared with 35% of patients whose D15.5% was above the median (). No other specific dosimetric indices for the PTV, bladder, bladder neck, or prostatic urethra were significantly associated with increased toxicity as defined in this study.
On multivariable analysis, both prostatic volume (OR 1.02, 95% CI 1–1.04, p = 0.019) and D15.5% to the bladder wall (OR 1.62, 95% CI 1.02–2.59, p = 0.043) were confirmed as independent predictors of acute urinary symptom flare. Conversely, the bladder wall V14.2 Gy was not found to be an independent predictor of acute urinary symptom flare. In patients whose prostatic volume and D15.5% were both below median values, there was a 13% rate of acute urinary symptom flare, compared with a rate of 45% in patients whose prostatic volume and D15.5% were each above mean values ().
Discussion
As seen in prior studies of prostate SBRT, treatment was well tolerated, with a transient increase in urinary symptoms followed by a relatively quick return to baseline function. Notably, prostatic volume was a significant predictor of acute urinary morbidity in this cohort of patients, similar to patients undergoing brachytherapy [Citation18,Citation19]. No other clinical factors, including patient age, clinical tumor stage, D’Amico risk group stratification, or ADT usage were associated with a worsening in IPSS at 7 d following treatment. Unexpectedly, baseline IPSS was not found to be a predictor of symptom flare, although this may be attributable to aggressive prophylactic alpha-adrenergic antagonist usage.
Comprehensive analysis of OAR dosimetry and its effect on short-term urinary morbidity revealed the dose to the hottest 15.5% of the bladder wall as a statistically significant predictor of acute urinary toxicity. Although bladder neck dose has been implicated in early urinary morbidity following low-dose rate (LDR) brachytherapy [Citation15], we were unable to demonstrate such a dose effect on this anatomical structure following SBRT. Surprisingly, the median maximum point dose in this cohort was higher in the bladder wall than in the bladder neck (41.3 versus 40.6 Gy). Contouring of the bladder neck is prone to error without a Foley catheter [Citation15], and its position is subject to change – suggesting the automatically generated bladder wall contour may be more reproducible and therefore more robust in predicting toxicity.
Interestingly, we were unable to demonstrate a relationship between the prostatic urethral dose and acute urinary toxicity, which has been observed in patients undergoing brachytherapy [Citation20,Citation21]. While robotic prostate SBRT planning is typically more inhomogenous than a comparable isocentric IMRT plan [Citation22], hot spots are considerably lower than those seen in brachytherapy plans, which may explain the lack of clear effect on urinary toxicity. Previously published results suggest that hot-spot avoidance in the prostatic urethra, in conjunction with prophylactic alpha-adrenergic antagonist use, may mitigate overall urinary bother and decrease the incidence of a late urinary symptom flare [Citation9,Citation23], highlighting the structure’s potential role in late, rather than acute, toxicity.
Our data suggest that larger prostate size and radiation hot spots in the bladder wall are associated with acute urinary morbidity, but it is unclear what effect these parameters will have on long-term urinary function. Nonetheless, minimizing hot spot placement in the bladder wall, a volume that can be easily generated from the whole bladder contour, is a relatively simple step to decrease urinary morbidity in prostate SBRT patients. Further follow-up of this cohort will be required to determine the applicability of these findings to late urinary toxicity following SBRT for localized prostate cancer.
IONC_1299221_Supplemental_material.docx
Download MS Word (453.9 KB)Disclosure statement
Sean P. Collins and Brian T. Collins are clinical consultants for Accuray.
References
- Lischalk JW, Kaplan ID, Collins SP. Stereotactic body radiation therapy for localized prostate cancer. Cancer J Sudbury J. 2016;22:307–313.
- Pollack A, Walker G, Horwitz EM, et al. Randomized trial of hypofractionated external-beam radiotherapy for prostate cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2013;31:3860–3868.
- Arcangeli S, Strigari L, Gomellini S, et al. Updated results and patterns of failure in a randomized hypofractionation trial for high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2012;84:1172–1178.
- Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17:1047–1060.
- Lee WR, Dignam JJ, Amin MB, et al. Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2016;34:2325–2332.
- Katz AJ, Kang J. Stereotactic body radiotherapy as treatment for organ confined low-and intermediate-risk prostate carcinoma, a 7-year study. Front Oncol. 2014;4:240.
- Katz AJ, Kang J. Quality of life and toxicity after SBRT for organ-confined prostate cancer, a 7-year study. Front Oncol. 2014;4:301.
- Yu JB, Cramer LD, Herrin J, et al. Stereotactic body radiation therapy versus intensity-modulated radiation therapy for prostate cancer: comparison of toxicity. J Clin Oncol. 2014;32:1195–1201.
- Repka MC, Guleria S, Cyr RA, et al. Acute urinary morbidity following stereotactic body radiation therapy for prostate cancer with prophylactic alpha-adrenergic antagonist and urethral dose reduction. Front Oncol. 2016;6:122.
- Kotecha R, Djemil T, Tendulkar RD, et al. Dose-escalated stereotactic body radiation therapy for patients with intermediate- and high-risk prostate cancer: initial dosimetry analysis and patient outcomes. Int J Radiat Oncol Biol Phys. 2016;95:960–964.
- Kole TP, Tong M, Wu B, et al. Late urinary toxicity modeling after stereotactic body radiotherapy (SBRT) in the definitive treatment of localized prostate cancer. Acta Oncol. 2016;55:52–58.
- Musunuru HB, Davidson M, Cheung P, et al. Predictive parameters of symptomatic hematochezia following 5-fraction gantry-based SABR in prostate cancer. Int J Radiat Oncol Biol Phys. 2016;94:1043–1051.
- Elias E, Helou J, Zhang L, et al. Dosimetric and patient correlates of quality of life after prostate stereotactic ablative radiotherapy. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2014;112:83–88.
- Cheung MR, Tucker SL, Dong L, et al. Investigation of bladder dose and volume factors influencing late urinary toxicity after external beam radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2007;67:1059–1065.
- Hathout L, Folkert MR, Kollmeier MA, et al. Dose to the bladder neck is the most important predictor for acute and late toxicity after low-dose-rate prostate brachytherapy: implications for establishing new dose constraints for treatment planning. Int J Radiat Oncol Biol Phys. 2014;90:312–319.
- Ghadjar P, Zelefsky MJ, Spratt DE, et al. Impact of dose to the bladder trigone on long-term urinary function after high-dose intensity modulated radiation therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2014;88:339–344.
- Lei S, Piel N, Oermann EK, et al. Six-dimensional correction of intra-fractional prostate motion with CyberKnife stereotactic body radiation therapy. Front Oncol. 2011;1:48.
- Raleigh DR, Chang AJ, Tomlin B, et al. Patient-and treatment-specific predictors of genitourinary function after high-dose-rate monotherapy for favorable prostate cancer. Brachytherapy 2015;14:795–800.
- Roeloffzen EM, van Vulpen M, Battermann JJ, et al. Pretreatment nomogram to predict the risk of acute urinary retention after I-125 prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2011;81:737–744.
- Zelefsky MJ, Yamada Y, Marion C, et al. Improved conformality and decreased toxicity with intraoperative computer-optimized transperineal ultrasound-guided prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2003;55:956–963.
- Ghadjar P, Matzinger O, Isaak B, et al. Association of urethral toxicity with dose exposure in combined high-dose-rate brachytherapy and intensity-modulated radiation therapy in intermediate- and high-risk prostate cancer. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2009;91:237–242.
- Hossain S, Xia P, Huang K, et al. Dose gradient near target-normal structure interface for nonisocentric CyberKnife and isocentric intensity-modulated body radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2010;78:58–63.
- Woo JA, Chen LN, Bhagat A, et al. Clinical characteristics and management of late urinary symptom flare following stereotactic body radiation therapy for prostate cancer. Front Oncol. 2014;4:122.
