Abstract
Background/objectives: Evaluation of our surveillance program for soft tissue sarcomas (STS) and borderline tumors (BT) for identification of local recurrence and lung metastases the first 2 years postoperatively.
Methods: We retrospectively assessed the medical files of all patients (n = 232) with STS and BT of the extremities and trunk wall who underwent surgery from 2010 to 2013. Two-hundred-and-thirty-two patients were included in the local recurrence study and 116 patients in the lung metastasis study. We extracted information on how local recurrence and lung metastases were detected. Kaplan–Meier survival analysis and 2 × 2-contingency table with Chi-square test were used. Local recurrence and lung metastases were analyzed separately.
Results: Twenty-five of 232 patients experienced local recurrence and 19 of 116 patients experienced lung metastases. Compared to clinical examination, local imaging led to a larger amount of local recurrence suspicions (37/560 vs. 8/706). Suspicions occurring on local imaging were more accurate than on clinical examination (17/37 vs. 0/8 affirmed). Local imaging identified a larger amount of local recurrence than clinical examination (17/560 vs. 0/706). Thirty-three patients suspected local recurrence themselves, 8 were affirmed. Compared to x-ray, computerized tomography (CT) led to a larger amount of lung metastasis suspicions (22/284 vs. 6/276). Suspicions occurring on CT seemed more accurate than on x-ray (15/22 vs. 2/6 affirmed). CT found a larger amount of lung metastases than x-ray (15/284 vs. 2/276). Three patients suspected lung metastases themselves, 1 was affirmed.
Conclusion: Bi-annual local imaging and CT the first 2 years after surgery of STS detect local recurrence and lung metastases better than clinical examination and x-ray. Clinical examination and x-ray between these examinations is unnecessary. Patients’ own suspicion of local recurrence and lung metastases is still important.
Introduction
Soft tissue sarcomas (STS) are a group of rare heterogeneous tumors, accounting for less than 1% of all malignant tumors [Citation1]. Approximately, 75% of all STS are located in the extremities, and the rest is spread between trunk wall, head and neck and retroperitoneal location [Citation2]. In Denmark, STS are histologically divided in three different malignancy grades according to the FNCLCC grading system; grade 1 = low malignant, grade 2 + 3 = high malignant [Citation3]. The standard treatment of localized STS in adult patients mainly consists of surgical wide excision. In marginally or intralesionally excised deep-seated tumors, surgery is often followed by external radiation therapy [Citation2,Citation4]. Local recurrence is seen in approximately 7–18% of patients, and metastases, primarily to the lungs, in 14–28% of patients [Citation5–9].
When local recurrence occurs, the options for new limb-sparring surgery, sometimes combined with radiotherapy, is accompanied with good local disease control [Citation7,Citation10]. Furthermore, even when lung metastases occur, it has been shown that surgical removal of the metastases is associated with improved survival, compared to patients who do not undergo metastasectomy [Citation11–14]. Most local recurrences and systemic disease, especially in high malignant tumors, appear within the first 2 years after initial surgery [Citation15–17]. This suggests that a more intense surveillance should be carried out during these years.
The current routine follow-up policy lacks evidence; there are few published datasets and no prospective studies. There is a broad consensus about a long-term follow-up for patients treated for STS, but the fact is that surveillance is time consuming and resource intensive, combined with the clear absence of prospective trials within this field, an agreement on the frequency of follow-up and what methods to use is absent [Citation5,Citation6,Citation18–23]. The existing strategies used for surveillance of STS are based on knowledge about the disease, treatment options and international guidelines; but even the guidelines lack specific recommendations, and are mostly based on a consensus rather than randomized controlled trials [Citation4,Citation24–26]. The majority of guidelines recommend a follow-up interval 3–4 times per year the first years post surgery, but consensus on which techniques should be used is absent [Citation4,Citation16,Citation24–27]. Clinical examination of the local tumor site and only magnetic resonance imaging (MRI) on suspicious findings, combined with chest x-ray for identification of lung metastases, is suggested most often [Citation5,Citation6,Citation16,Citation21,Citation23,Citation27]. In the latest clinical guidelines by the European Society for Medical Oncology [Citation4], it is mentioned that a combination of focal MRI and chest computerized tomography (CT) scan of the lungs could be used to identify local recurrence and lung metastases, although the benefits and cost-effectiveness of such a program is still to be established.
In January 2010, we introduced a new follow-up program for the first two postoperative years in our orthopedic departments with the aim of identifying local recurrence and lung metastases after primary surgical excision of STS and borderline tumors (BT) of the extremities and trunk wall.
In the new standardized surveillance program, patients with intermediate- and high grade STS were examined four times a year, alternating between a clinical examination preceded by regular chest x-ray and a clinical examination preceded by focal MRI and low-dose chest CT (without contrast). For some histological subtypes (synovial-, clear cell-, epithelioid- and angiosarcomas), a few exceptions were made. Here, total body positron emission tomography combined with CT (PET/CT) or CT examination of thorax and abdomen (myxoid liposarcomas) was used.
Low malignant STS and BT were examined four times a year, alternating between just a clinical examination and a clinical examination proceeded by a focal MRI. Both the chest x-ray and CT were not included in the schedule for low malignant tumors. The examinations, including MRI and chest CT, were intended for earlier detection of local recurrence and lung metastases. The aim of this study was to evaluate the effect of our new follow-up program including how and with what techniques local recurrence and lung metastases are detected.
Patients and methods
Study design and data collection
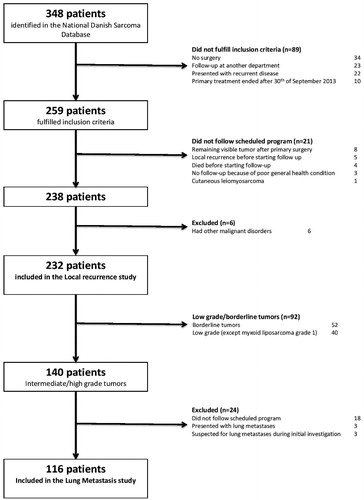
Using the National Danish Sarcoma Database [Citation28], we identified all patients (n = 348) (age >16 years) treated with primary surgery for STS (including BT) of the extremities or trunk wall from 1 January 2010 to 30 September 2013 and who had follow-up at the Departments of Orthopedic Surgery, Rigshospitalet, Copenhagen and Odense University Hospital. We retrospectively assessed all patient files and patients who did not fulfill the above-mentioned inclusion criteria (n = 89), patients who did not follow the scheduled surveillance program (n = 21) and patients who had other malignant disorders were excluded (). Two hundred and thirty-two patients ended up in our local recurrence cohort and were included in the part of the study regarding local recurrence (). For the part of the study regarding lung metastases, we further excluded low grade and BT (n = 92) because of their low potential to metastasize (). We excluded patients who presented with or were suspected to have lung metastases and patients who did not follow the scheduled program (n = 24) (). Finally, 116 patients ended up in our lung metastasis cohort (). The study was approved by the Danish Data Protection Agency (j. no. 2014-41-3489) and the Danish Health and Medicine Authority (j. no. 33013868/1 and 33013868/2).
Table 1. Patient demographics – local recurrence.
Table 2. Patient demographic – lung metastasis.
Patient population
From the medical records, patient gender, age at time of surgery, tumor size, location and depth and presence of metastatic lesions were obtained. From the Danish National Pathology Registry (DNPR) [Citation29] we found histopathological diagnosis, tumor malignancy grade and surgical margin (classified as wide, marginal or intralesional). Patient and tumor characteristics for the two cohorts are listed in and .
For each patient, outpatient contacts the first 25 months post surgery were reviewed in order to examine how local recurrences and metastases were detected during these years. Only outpatient contacts and radiologic examinations scheduled for regular follow-up were included. Additional imaging or clinical controls because of suspicious findings, either on clinical or previous radiologic examinations, were all excluded. We used the original radiologic specialist evaluations of all radiographs and scans included in the study. The result of each surveillance technique was recorded as either normal or suspicious for local recurrence or metastases. All suspicions were then subject to further examination, including more imaging diagnostics and biopsy, and finally classified as affirmed or refuted.
Statistical analysis
Descriptive data are presented as mean and range. Local recurrence and lung metastases were analyzed separately. The time from surgery to either biopsy- or image-proven local recurrence or lung metastases was calculated, and a Kaplan–Meier analysis was applied to estimate the total cumulative 25-month local recurrence-free and metastasis-free survival of the two cohorts. The total survival time of the first 25 months was the time from surgery until local recurrence, lung metastases or death.
To find out how and with what techniques we identified local recurrences and lung metastases, we applied 2 × 2 contingency tables with a Chi-square test to compare the different diagnostic tests. Focal MRI, focal CT, PET/CT and ultrasound were grouped as local imaging control and compared as one group to clinical examination. Low-dose chest CT, CT of the thorax and abdomen and PET/CT were grouped as CT-scans of the lungs and compared as one group to regular chest x-ray.
Confidence interval is reported as 95% confidence intervals (95% CI) and p values <.05 are considered statistical significant. The statistical analysis was performed using STATA software version 13.0 (StataCorp, College Station, TX, USA) for Mac and SPSS software version 20 (SPSS Inc., Chicago, IL, USA) for Windows.
Results
Local recurrence
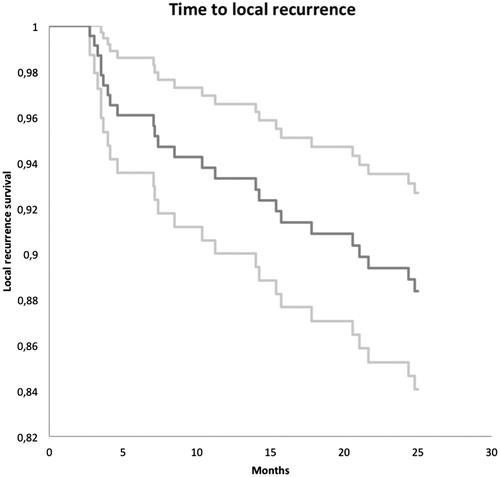
In total, we included 232 patients in our study regarding local recurrence. Twenty-five of them experienced local recurrence within the first 25 months post surgery, corresponding to a 25 months local recurrence free rate of 88% (). At the time of the last follow-up, 214 patients in total were alive and 18 had died. During the study period, additional 34 patients had follow-up less than 25 months after surgery. Twenty-one of these patients started follow-up at another department, 5 patients repeatedly failed to attend imaging and clinical controls, 3 patients did not want to attend follow-up, 3 patients were lost for other reasons and 2 patients died. Mean time of follow-up for the entire group was 21.4 months (0.3–25). Mean time to local recurrence was 11.9 moths (2.7–24.8), and the mean time of follow-up for the group of patients with no recurrence was 22.7 months (0.3–25).
Figure 2. Kaplan–Meier curve showing the cumulated local recurrence free rate (dark grey) and corresponding 95% CI (light grey).
Surveillance methods
The first two years following treatment for STS, in total, the patients underwent 706 clinical examinations and 560 local imaging examinations of the local tumor site, defined as 521 MRIs, 24 PET/CTs, 11 focal CTs and 4 ultrasound examinations. The 11 CTs were performed instead of MRI, because the patients had either a pacemaker or an orthopedic implant in the region of interest, and four ultrasounds because of adiposity. Included in these figures are only examinations performed as a part of a regular follow-up. Of the 25 local recurrences, 17 (68%) were found by local imaging, 8 recurrences (32%) by the patients themselves, and 0 (0%) at clinical examination. In 10 out of the 17 local recurrences found on local imaging, the clinician was not able to palpate a potential tumor at the following clinical examination. In three cases, the clinicians were able to palpate a tumor and in four cases, no information was found in the patient files. Compared to clinical examination, local imaging (mainly MRI) led to a statistically significant larger amount of suspicions of local recurrence (37/560 vs. 8/706, p < .0005). Furthermore, the suspicions occurring on local imaging were more accurate than suspicions occurring on clinical examination (17/37 affirmed vs. 0/8 affirmed, p = .015). The 25 suspicions of local recurrence were affirmed with histology (biopsy/tumor excision = 12/6) or additional/new local imaging = 7.
Comparing the total number of examinations performed, local imaging identified a statistically significant larger amount of local recurrences than clinical examination did (17/560 (3%) vs. 0/706 (0%), p < .0005).
Thirty-three patients suspected local recurrence themselves, 8 of these were affirmed. In these 8 cases, 1 patient contacted our clinic with a suspicion before the first scheduled control, in 4 cases, the patients showed up with the suspicion either at the first scheduled control or shortly after, and in 3 cases, the patients came after the third scheduled control or later.
The local recurrences were treated with surgery in 15 cases, with chemotherapy in 4 cases, with surgery and radiotherapy in 3 cases, with radiotherapy alone in 1 case, pending approach in 1 case and no treatment in 1 case. Six of the 8 patients (75%) who discovered recurrence themselves had died at the time of the last follow-up session. Of the 17 local recurrences found on local imaging, only 2 (12%) had died at the time of last follow-up.
Lung metastases
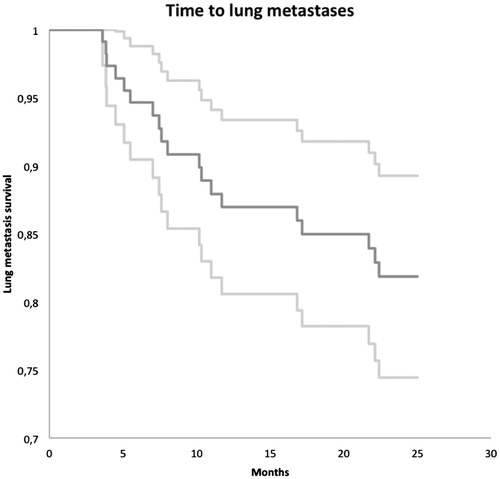
In total, we included 116 patients in our study regarding lung metastases. Eighteen experienced lung metastases within the first 25 months post surgery, corresponding to a 25 months metastasis free rate of 82% (). During the study period, 20 patients were lost to follow-up. Fifteen patients started follow-up at another department, 1 patient repeatedly failed to attend imaging and clinical examinations, 2 patients were lost for other reasons and 2 patients died. At the time of last follow-up, in total, 101 patients were alive and 15 had died. Mean time of follow-up for the entire group was 20.2 months (0.6–25), mean time to lung metastases was 10.5 months (3.6–22.4) and mean time of follow-up for the group of patients with no lung metastases was 22.1 months (0.6–25).
Figure 3. Kaplan–Meier curve showing the cumulated lung metastasis free rate (dark grey) and corresponding 95% CI (light grey).
Surveillance methods
The first 2 years following treatment for STS, the patients in total underwent 276 x-rays and 284 CT-scans of the chest. Only examinations scheduled for regular follow-up are included in these numbers. Of the 18 lung metastases, 15 (79%) were found by CT, 2 metastases (10.5%) by x-ray and 1 (5%) because of symptoms reported from the patient. Compared to x-ray, CT led to a statistically significant larger amount of suspicions of lung metastases (22/284 vs. 6/276, p = .002). Furthermore, the suspicions occurring on CT seemed to be more accurate than suspicions occurring on x-ray (15/22 affirmed vs. 2/6 affirmed, p = .121). Only in 2 cases, an x-ray finding of lung metastases was correct in which an x-ray was the first chest examination after surgery and radiotherapy. One suspicion on CT were neither affirmed nor denied because of loss to follow-up. Three patients suspected lung metastases themselves and 1 of them was affirmed. The 18 suspicions of lung metastases were affirmed by histology (tumor excision/biopsy = 12/2) or additional CT = 4. Comparing the total number of examinations performed, CT found a larger amount of lung metastases than x-ray did (15/284 (5.3%) vs. 2/276 (0.7%), p = .002). The lung metastases were treated with surgery (n = 12), with chemotherapy (n = 4) and with surgery combined with radiotherapy (n = 2). The 2 patients on whom lung metastases were found on x-ray had both died at the time of last follow-up (100%). Of the 15 patients where CT-scan discovered lung metastases, 10 (67%) had died at the time of last follow-up. In addition to the 19 lung metastases, 12 patients experienced non-pulmonary metastases during follow-up: two subcutaneous, 2 skeletal, 2 lymphatic, 1 cerebral, 1 muscular, 1 in the breast, 1 axillary, 1 in the elbow area and 1 in the liver.
Discussion
We found that in our early follow-up program covering the first 2 post-operative years, local imaging (mostly MRI) identifies a larger amount of local recurrences than clinical examinations. It seems that local imaging is superior to regular clinical examination in early detection of local recurrences in a population consisting of a mixture of patients surgically treated for STS of both high- and low-malignant (including BT) histological types. We also found that CT-scans identified a larger amount of true lung metastases compared to plain x-ray. Thus, CT seems to be the most useful method for early detection of lung metastases in high-malignant STS.
To our knowledge, this study is the only existing study where all patients have been in a follow-up program in which both kinds of surveillance modalities (local imaging vs. clinical examination and CT vs. x-ray) have been scheduled for each patient. This allowed us to compare the different surveillance methods on a very uniform basis. Given that a follow-up regime is often selected on the basis of individual patient and tumor characteristics, we eliminated that bias by only taking into account the tumor and histologic grade and here by assigning all patients to a predetermined follow-up protocol.
Several other studies have examined the role of follow-up and what kind of surveillance method to use. Reviewing the literature, the majority recommend clinical examination and regular x-ray of the lungs 3–4 times a year, the first 2 years post surgery, and many studies also state that patients often find local recurrences themselves [Citation5,Citation16,Citation19,Citation21–23,Citation27]. This is contrary to our results that show no local recurrences are found at clinical examination and CT seems to be superior to regular x-ray in finding lung metastases.
In our surveillance program, a focal MRI preceded the clinical examination at every second visit. One could suggest that the local recurrence found on MRI also would be found on the following clinical examination. In our study, the clinicians were only able to palpate a potential tumor in 3 out of 17 local recurrences found on local imaging, and in 10 cases, nothing could be palpated. It is important to consider the fact that the clinician may be biased, already knowing that a local recurrence is present at the tumor site. Furthermore, differentiating between a local recurrence or surgery/radiation-induced scar tissue could be difficult at a regular clinical examination, and in the end, a local imaging would be needed for conclusion. It is important mentioning, that our study is designed to show if the follow-up program used in the present population finds local recurrences and not designed to show that clinical examination does not work in other follow-up programs.
Rothermundt et al. [Citation5] assessed 174 patients, retrospectively, all treated for primary STS. They reported that 22 local recurrences were found by self-examination, 8 by clinical examination and only in 1 case MRI detected recurrence. Lung metastases were detected by x-ray in 19 asymptomatic patients, were incidental findings on 3 CT-scans, and 6 patients had symptomatic metastases shortly after primary treatment. The authors conclude that x-ray appears to be sufficient in finding lung metastases and that clinical examination is a more effective method than MRI for local recurrence. These results should be interpreted with caution since the follow-up program did not include regular CT and only 1 MRI, 6 months post surgery. Thus, the chance of finding recurrences, both local and distant, on MRI and CT would consequently be very small.
Another retrospective study by Cool et al. [Citation21] examined a follow-up program, also consisting of clinical examination and x-ray, and concluded that x-ray finds most asymptomatic lung metastases, while clinical examination only finds one-third of local recurrences. Of the 40 lung metastases, 30 were identified on x-ray, and 25/30 (83%) patients had died at the end of the study period. A more aggressive surveillance method, like CT, might have found lung metastases earlier, and, thereby, improved overall survival. The authors suggest that using a more sophisticated imaging modality of the primary tumor site could detect more small local recurrences. In our study, 68% of local recurrences were detected on local imaging and only 32% were symptomatic and found by patients.
Watts et al. [Citation30] sought to find out the value of MRI as a screening tool in tumor surveillance. A cohort consisting of 57 musculoskeletal tumors were included in the study. Nine of 13 (69%) recurrences were detected on a scheduled surveillance scan and 4 of 13 (31%) on scans in-between. The authors are aware of the small heterogeneous cohort, but still conclude that MRI has a role in early detection of STS.
Cho et al. [Citation18] compared two matched groups with high-grade STS, retrospectively, who were followed with either x-ray or CT. There was an equivalent distribution of metastases in the two cohorts, but the metastases in the CT cohort had the tendency of unilaterality, smaller size, fewer numbers, more often treated with metastasectomy and markedly better survival rates than the patients in the x-ray cohort. The authors found a 2- and 4-year survival rate after detection of lung metastases at 20.1 and 0% in the x-ray cohort and at 47.4 and 31.6% in the CT cohort, although no significant difference in overall 5-year survival is found. Based on this and the given fact that surgical metastasectomy is the only potentially curative therapy, they concluded that serial monitoring with CT-scans could eventually lead to a survival advantage.
Factors associated with survival outcome after pulmonary metastasectomy of STS metastases have been investigated in several studies [Citation12,Citation14,Citation31,Citation32]. The ability to perform a complete resection (R0) of all lung metastases compared to incomplete (R1/R2) was significantly associated with improved overall survival by Weiser et al. [Citation12], Smith et al. [Citation31] and Pfannschmidt et al. [Citation14]. From a technical view, patients with incomplete resection tend to have bilateral disease or multiple pulmonary metastases, here by adding to the findings of Cho et al. [Citation18], that CT would lead to improved survival. Another study by Garcia Franco et al. [Citation32] also finds that a small number of lung metastases seem to be significantly associated with better survival. All these support that early recognition of lung metastases seems to be preferable.
Christie-Large et al. [Citation33] investigated imaging modalities for lung metastases in STS-patients at initial presentation. They reported specificity and sensitivity for CT and x-ray at 99.6 and 100% vs. 99.6 and 60.5%, respectively. The authors showed by using only x-ray for cancer staging, lung metastases would be missed in 36 patients, mostly high-grade, large tumors, corresponding to 37.5% of patients where CT would have found it, here by confirming that CT will detect a greater number of lung metastases.
Also, the consequences of radiation exposure from surveillance CT are increasingly debated. Several reports mention the risk of radiation-induced cancers [Citation34–36] accompanied with the increasing use of CT. Is the benefits of finding asymptomatic nodules affordable compared to the radiation exposure? In our follow-up program, only low-dose chest CT is used exposing the patient for a radiation dose at maximum 2 mS, and not a regular CT (radiation dose = 7 mS) [Citation37]. Suspicions of lung metastases on a regular chest x-ray (radiation dose = 0.1 mS) [Citation38] or a digital tomosynthesis of the chest (radiation dose =0.2 mS) [Citation39] would typically require an additional CT to plan the following biopsy or tumor excision. Furthermore, the digital tomosynthesis is more limited in the depth of field than a CT is [Citation39], but nevertheless it might be a useful alternative to CT for the detection of lung metastases.
Large multicenter prospective studies are needed to investigate the actual benefits of a more intense surveillance, including patients’ acceptance, outcome on long-term survival, exposed radiation dose and cost-effectiveness.
Very few studies have been published assessing patients’ expectations or the acceptability of post-treatment follow-up strategies. Even the above-mentioned studies regarding the optimal follow-up program do not take the patients’ experience and expectations into account, including our study. Accompanying the benefits of identifying local and distant recurrent disease is the risk of false-positive findings and the increased testing. In our study, both MRI and CT lead to a significantly larger amount of suspicions of local and distant disease than clinical examination and x-ray did. However, the suspicions seemed to be more accurate than clinical examination and x-ray, advocating no difference in the perceived fear of false-positive results, when using a more intense surveillance program.
In a British study [Citation40], 132 patients were asked about the most important aspects of a surveillance program following STS-surgery and also the acceptability of the program in context with different levels of recurrence risk. Clinical examination and x-ray every 6 months for 5 years was the most valued, but with increasing recurrence risk, the request for a more intensive surveillance including MRI and CT increased. At 5% recurrence risk, the intensive surveillance program became the preferred choice. Considering the recurrence risk for STS varies between the aforementioned 7–18%, the study supports a high intensive follow-up program when recurrence risk is considered. The authors mention ‘the lure of the familiar’ as the reason most patients prefer clinical examination and x-ray regime when not considering the recurrence risk.
One study has investigated the anxiety referring to surveillance CT in long-term lymphoma survivors [Citation41] and found a 37% incidence of clinically significant anxiety symptoms and a fear of recurrence that became remarkably prevalent in the time before surveillance scans and did not decrease with increasing years since diagnosis. This again illustrates the importance of the ability to balance multiple objectives, including maximizing patient survival, quality of life, psychological outcome, physical function and economics. Studies supporting a less intensive follow-up discusses, amongst other things, the anxiety associated with an unnecessary amount of surveillance scans. Robust evidence of improvement in long-term survival following a more intense surveillance program is needed. Our study only examines the first 2 years post surgery and furthermore the study is not designed to give information about differences in long-term survival regarding local recurrences and lung metastases according to the technique that discovers recurrent disease. For investigation of long-term survival, both a much larger group of patients and a different study design including a control group would be needed. However, it is noticeable that both patients with metastases found on x-ray died in our follow-up period and only 10/15 in the CT cohort died. Furthermore, we found that 6 out of 8 local recurrences discovered by patients themselves had died at the time of last follow-up, and only 2 out of 17 local recurrences found on local imaging had died at that time. However, this might by fully expected since the first group probably has a faster growing tumor and therefore a more aggressive biology than those found on local imaging.
Another important aspect is the cost of surveillance in STS. Following the relatively different surveillance strategies between departments, tremendous variation in cost is seen. Goel et al. [Citation42] quantified the Medicare-allowed costs associated with various published five-year surveillance programs between 1982 and 2003. They found a 42.8-fold cost differential ($485–21.235) here by emphasizing the importance of investigation of cost-effectiveness attached to different programs.
This study has limitations. Even though the retrospective nature of the study could have influenced the clinicians’ decision of what kind of surveillance group the patients entered, our standardized program secured that all patients were allocated based on very specific factors such as tumor grade and histological diagnosis. Our patient group only includes primary STS (and BT) with no metastatic disease at presentation, and therefore a more homogeneous group than studies including both bone sarcoma and STS, recurrent and primary tumors and differences in distant disease. Furthermore, we excluded all low-malignant tumors from the lung metastasis cohort, making it even more homogenous.
The cohort consists of 22 different histopathological subtypes. The difference of the nature of tumors has not been considered because of the rareness of the disease in general. Therefore, we are limited to a generalized conclusion regarding optimal follow-up for the entire group.
As previously mentioned, we did not follow our patients long enough to conclude anything about the benefit of earlier detection of recurrent disease and impact on long-term survival.
Conclusion
This study regarding STS and BT in adult patients above 16 years of age shows that local imaging finds a significantly larger amount of local recurrences than clinical examination, compared to the amount of each examination performed. This leads to the conclusion that local imaging seems to be the most useful method in early detection of local recurrence in patients surgically treated for both STS and BT. Furthermore, we showed that CT seems to be the most useful method for early detection of lung metastases, because CT identified a significantly larger amount of real lung metastases compared to plain x-ray.
Subsequent to these findings, we consider changing our follow-up program by removing all x-rays and clinical examinations between the 6 monthly visits the first 2 years post surgery with local imaging (and if high-malignant tumor CT). We suggest high-malignant tumors should be followed every 6 months with MRI, CT and clinical examination and low-malignant tumors every 6 months with MRI and clinical examination. Both groups should have a clinical examination at 3 months post surgery and in cases with high-malignant histology a lung x-ray or CT should be added. At all times, patients may contact the clinic if symptomatic, hereby including patients’ own suspicions of local recurrence and lung metastases.
Disclosure statement
Authors declare no conflicts of interest.
Additional information
Funding
References
- NORDCAN project [Internet]. 2013 [cited 2015 14.12.2015]; Available from: http://www-dep.iarc.fr/NORDCAN/English/frame.asp
- Fletcher CDM, Bridge JA, Hogendoorn PW, et al. World Health Organisation classification of tumours of soft tissue and bone. 4th ed. Lyon: International Agency for Research on Cancer (IARC); 2013.
- Trojani M, Contesso G, Coindre JM, et al. Soft-tissue sarcomas of adults; study of pathological prognostic variables and definition of a histopathological grading system. Int J Cancer. 1984;33:37–42.
- Group EESNW. Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii102–iii112.
- Rothermundt C, Whelan JS, Dileo P, et al. What is the role of routine follow-up for localised limb soft tissue sarcomas? A retrospective analysis of 174 patients. Br J Cancer. 2014;110:2420–2426.
- Cheney MD, Giraud C, Goldberg SI, et al. MRI surveillance following treatment of extremity soft tissue sarcoma. J Surg Oncol. 2014;109:593–596.
- Potter BK, Hwang PF, Forsberg JA, et al. Impact of margin status and local recurrence on soft-tissue sarcoma outcomes. J Bone Joint Surg Am. 2013;95:e151.
- Sabolch A, Feng M, Griffith K, et al. Risk factors for local recurrence and metastasis in soft tissue sarcomas of the extremity. Am J Clin Oncol. 2012;35:151–157.
- Stojadinovic A, Leung DH, Hoos A, et al. Analysis of the prognostic significance of microscopic margins in 2,084 localized primary adult soft tissue sarcomas. Ann Surg. 2002;235:424–434.
- Catton C, Davis A, Bell R, et al. Soft tissue sarcoma of the extremity. Limb salvage after failure of combined conservative therapy. Radiother Oncol. 1996;41:209–214.
- Kim S, Ott HC, Wright CD, et al. Pulmonary resection of metastatic sarcoma: prognostic factors associated with improved outcomes. Ann Thorac Surg. 2011;92:1780–1786.
- Weiser MR, Downey RJ, Leung DH, et al. Repeat resection of pulmonary metastases in patients with soft-tissue sarcoma. J Am Coll Surg. 2000;191:184–190.
- Kandioler D, Kromer E, Tuchler H, et al. Long-term results after repeated surgical removal of pulmonary metastases. Ann Thorac Surg. 1998;65:909–912.
- Pfannschmidt J, Klode J, Muley T, et al. Pulmonary metastasectomy in patients with soft tissue sarcomas: experiences in 50 patients. Thorac Cardiovasc Surg. 2006;54:489–492.
- Sinha S, Peach AH. Diagnosis and management of soft tissue sarcoma. BMJ. 2010;341:c7170
- Kane JM. 3rd. Surveillance strategies for patients following surgical resection of soft tissue sarcomas. Curr Opin Oncol. 2004;16:328–332.
- Patel SR, Zagars GK, Pisters PW. The follow-up of adult soft-tissue sarcomas. Semin Oncol. 2003;30:413–416.
- Cho HS, Park IH, Jeong WJ, et al. Prognostic value of computed tomography for monitoring pulmonary metastases in soft tissue sarcoma patients after surgical management: a retrospective cohort study. Ann Surg Oncol. 2011;18:3392–3398.
- Puri A, Gulia A, Hawaldar R, et al. Does intensity of surveillance affect survival after surgery for sarcomas? Results of a randomized noninferiority trial. Clin Orthop Relat Res. 2014;472:1568–1575.
- Gerrand CH, Billingham LJ, Woll PJ, et al. Follow up after primary treatment of soft tissue sarcoma: a survey of current practice in the United Kingdom. Sarcoma. 2007;2007:34128
- Cool P, Grimer R, Rees R. Surveillance in patients with sarcoma of the extremities. Eur J Surg Oncol. 2005;31:1020–1024.
- Johnson FE, Sakata K, Kraybill WG, et al. Long-term management of patients after potentially curative treatment of extremity soft tissue sarcoma: practice patterns of members of the Society of Surgical Oncology. Surg Oncol. 2005;14:33–40.
- Shikada Y, Yano T, Maruyama R, et al. Effective utilization of chest x-ray for follow-up of metastatic lung tumor due to soft tissue sarcoma. Ann Thorac Cardiovasc Surg. 2013;19:103–106.
- von Mehren M, Randall RL, Benjamin RS, et al. Soft tissue sarcoma, Version 2.2016. NCCN Clin Practice Guidelines Oncol. 2016;14:758–86.
- Grimer R, Judson I, Peake D, et al. Guidelines for the management of soft tissue sarcomas. Sarcoma. 2010;2010:506182.
- Roberts CC, Kransdorf MJ, Beaman FD, et al. ACR appropriateness criteria follow-up of malignant or aggressive musculoskeletal tumors. J Am Coll Radiol. 2016;13:389–400.
- Whooley BP, Gibbs JF, Mooney MM, et al. Primary extremity sarcoma: what is the appropriate follow-up? Ann Surg Oncol. 2000;7:9–14.
- Jørgensen PH, Lausten GS, Pedersen AB. The Danish Sarcoma Database. Clin Epidemiol. 2016;8:685–90.
- Bjerregaard B, Larsen OB. The Danish Pathology Register. Scand J Public Health. 2011;39:72–74.
- Watts AC, Teoh K, Evans T, et al. MRI surveillance after resection for primary musculoskeletal sarcoma. J Bone Joint Surg Br. 2008;90:484–487.
- Smith R, Pak Y, Kraybill W, et al. Factors associated with actual long-term survival following soft tissue sarcoma pulmonary metastasectomy. Eur J Surg Oncol. 2009;35:356–361.
- Garcia Franco CE, Algarra SM, Ezcurra AT, et al. Long-term results after resection for soft tissue sarcoma pulmonary metastases. Interact Cardiovasc Thorac Surg. 2009;9:223–226.
- Christie-Large M, James SL, Tiessen L, et al. Imaging strategy for detecting lung metastases at presentation in patients with soft tissue sarcomas. Eur J Cancer. 2008;44:1841–1845.
- Brenner DJ, Hall EJ. Computed tomography-an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284.
- Huppmann MV, Johnson WB, Javitt MC. Radiation risks from exposure to chest computed tomography. Semin Ultrasound CT MR. 2010;31:14–28.
- Berrington de Gonzalez A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071–2077.
- Larke FJ, Kruger RL, Cagnon CH, et al. Estimated radiation dose associated with low-dose chest CT of average-size participants in the national lung screening trial. AJR Am J Roentgenol. 2011;197:1165–1169.
- Lin EC. Radiation risk from medical imaging. Mayo Clin Proc. 2010;85:1142–1146.
- Gomi T, Nakajima M, Fujiwara H, et al. Comparison between chest digital tomosynthesis and CT as a screening method to detect artificial pulmonary nodules: a phantom study. Br J Radiol. 2012;85:e622–e629.
- Damery S, Biswas M, Billingham L, et al. Patient preferences for clinical follow-up after primary treatment for soft tissue sarcoma: a cross-sectional survey and discrete choice experiment. Eur J Surg Oncol. 2014;40:1655–1661.
- Thompson CA, Charlson ME, Schenkein E, et al. Surveillance CT scans are a source of anxiety and fear of recurrence in long-term lymphoma survivors. Ann Oncol. 2010;21:2262–2266.
- Goel A, Christy ME, Virgo KS, et al. Costs of follow-up after potentially curative treatment for extremity soft-tissue sarcoma. Int J Oncol. 2004;25:429–435.