Abstract
Background: Recurrence is a common outcome among patients that have undergone an intended curative resection for colorectal cancer. However, data on factors that influence colorectal cancer recurrence are sparse. We report descriptive characteristics of both colon and rectal cancer recurrence in an unselected population.
Material and methods: We identified 21,152 patients with colorectal cancer diagnosed between May 2001 and December 2011 and registered with the Danish Colorectal Cancer Group. Recurrences were identified in 3198 colon and 1838 rectal cancer patients during follow-up. We calculated the frequency, proportion, and incidence rates of colon and rectal cancer recurrence within descriptive categories, and the cumulative five- and ten-year incidences of recurrence, treating death as a competing risk. We used a Cox proportional hazard model to calculate hazard ratios (HR) and 95% confidence intervals (CI).
Results: Recurrence risk was highest in the first three years of follow-up. Patients <55 years old at initial diagnosis (incidence rate for colon: 7.2 per 100 person-years; 95% CI: 6.5–7.9; rectum: 8.1 per 100 person-years; 95% CI: 7.2–9.0) and patients diagnosed with stage III cancer (colon HR: 5.70; 95% CI: 4.61–7.06; rectal HR: 7.02; 95% CI: 5.58–8.82) had increased risk of recurrence. Patients diagnosed with stage III cancer from 2009 to 2011 had a lower incidence of recurrence than those diagnosed with stage III cancer in the years before. Cumulative incidences of colon and rectal cancer recurrence were similar for both cancer types among each descriptive category.
Conclusions: In this population, increases in colorectal cancer recurrence risk were associated with younger age and increasing stage at diagnosis. Cumulative incidence of recurrence did not differ by cancer type. Descriptive characteristics of colon and rectal cancer recurrence may help to inform patient–physician decision-making, and could be used to determine adjuvant therapies or tailor surveillance strategies so that recurrence may be identified early, particularly within the first 3 years of follow-up.
Introduction
Colorectal cancer is the third most common cancer in the world; the lifetime risk for developing colorectal cancer is ∼5% in both the United States and Denmark [Citation1,Citation2]. In 2012, about 1.4 million cases were diagnosed worldwide with 135,000 cases in the United States [Citation3] and 4800 in Denmark [Citation4]. Colorectal cancer prognosis largely depends on the stage at which it is diagnosed: at localized stage, treatment is most successful and the five-year survival rate is 90%. In contrast, the five-year survival rates for regional and distant stages are 70 and 13%, respectively [Citation3]. Currently, there are 1.2 million colorectal cancer survivors in the United States alone [Citation3]; of these survivors, recurrence is a major concern. Recurrence reflects disease burden and quality of care, including staging, surgery, and adjuvant therapies, and is itself a risk factor for subsequent disease progression and death. The European Society for Medical Oncology guidelines recommend adjuvant chemotherapy for some subgroups of colorectal cancer patients; however, this recommendation is based on older data that do not reflect recent improvements in preoperative staging and surgical techniques [Citation5]. Specific data on characteristics associated with increased recurrence risk could guide the type (e.g., oxaliplatin) and extent of adjuvant therapy in certain patient groups, and inform effective follow-up plans to detect cancer progression.
Population-wide recurrence data are, unfortunately, seldom available. Furthermore, studies of cancer incidence, recurrence, and mortality often combine colon and rectal sites, though they may have important differences, particularly in hypermutated tumors [Citation6–8]. We have recently validated an algorithm by which recurrence can be identified [Citation9]. Here, we apply this algorithm to an unselected population of stage I–III colon and rectal cancer patients to provide the first nationwide descriptive data on both colon and rectal cancer recurrence risk.
Material and methods
Data sources and data collection
Records in the Danish Colorectal Cancer Group (DCCG) database, the Danish National Registry of Patients (DNPR), Danish Cancer Registry (DCR), and Danish Pathology Registry (DPR) were linked by the Central Personal Registry number, a unique identifying number assigned to Danish citizens and legal residents. The DCCG database is nearly a complete clinical database of Danish colorectal cancer patients [Citation10–12]. In addition to the date of surgery, this database provided information on the date of colorectal cancer diagnosis as the date of the first hospital contact with a diagnosis of colon or rectal cancer, stage at diagnosis, and receipt of surgery and chemotherapy. The DNPR contains administrative and clinical data, including information on patient demographics, diagnoses, and surgical procedures, and has estimated 94% sensitivity and 100% specificity for classification of receipt of chemotherapy in colorectal cancer patients [Citation13,Citation14]. Data from the DNPR also informed our measure of prevalent comorbid diseases at the time of colorectal cancer diagnosis. The DCR is a population-based registry that contains all incidences of malignant neoplasms from 1943 onwards, and includes patient and tumor characteristics [Citation15]. The DPR, which contains electronically recorded standard data on biological specimens from all Danish pathology departments since 1998, was used to identify pathologically diagnosed recurrences [Citation16]. The study protocol was approved by the Danish Data Protection Agency (record number 2011–41–6968) and the North Denmark Region Committee on Health Research Ethics (record number N–20130027). Registry-based research in Denmark does not require participants’ informed consent.
Study population
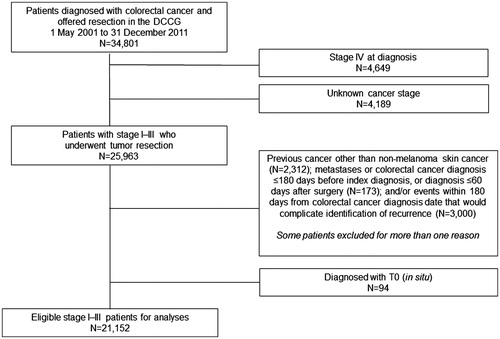
We included Danish colon and rectal cancer patients who had surgery and were registered in the DCCG database between 1 May 2001 and 31 December 2011. We excluded patients with metastatic disease and unknown cancer stage. We also excluded patients with a diagnosis of colorectal cancer or metastases in the DNPR or DCR within 180 days of the date of colorectal cancer diagnosis as recorded in the DCCG database, and excluded patients with a diagnosis of cancer different from colorectal cancer (except non-melanoma skin cancer) or cancer metastases before the date of initial colorectal cancer diagnosis. Patients were followed to December 2012.
Definition of analytic variables
Cohort characteristics
Eligible patients were categorized as having colon or rectal cancer. Rectal cancers were clinically defined as those 0–15 cm from the anus. Within these cancer types, patients were categorized by sex, age group at cancer diagnosis (<55, 55–64, 65–74, 75–84, or ≥85 years old), AJCC stage at diagnosis (I, II, or III) [Citation17], Charlson comorbidity score [Citation18] at diagnosis (0, 1 or 2, or ≥3), surgical procedure, number of lymph nodes with metastases (0, 1–3, 4–6, or ≥7), calendar period of cancer diagnosis (2001–2004, 2005–2008, or 2009–2011), surgical approach (laparotomy, laparoscopy, or endoscopy), and surgical urgency (elective or acute). Surgeries converted from laparoscopy to laparotomy were categorized as the latter; endoscopies were mucosal resections or transanal endoscopic microsurgeries. Data on utilization of neoadjuvant and adjuvant therapy were available for patients diagnosed with colon or rectal cancer from 2009 to 2011.
Recurrences
To identify colon and rectal cancer recurrences, we used a previously-developed algorithm with sensitivity of 95%, specificity of 97%, positive-predictive value of 86%, and negative-predictive value of 99% when compared to an actively followed cohort of colorectal cancer patients [Citation9]. We defined colon or rectal cancer recurrences as (a) tumor growth at or near the site of the original tumor and in the same organ (colon or rectum), or (b) metastases to tissue adjacent to the original tumor site or to a distant organ. We identified recurrences as having codes for metastasis, cytostatic therapy, or Systematized Nomenclature of Medicine (SNOMED) combinations corresponding to malignant biopsies recorded 180 or more days after first colorectal cancer surgery, 60 or more days after last cytostatic therapy code, and without a new primary cancer diagnosis registered in DNPR or DCR between the date of the first colorectal cancer surgery and the date of the DNPR cytostatic therapy code [Citation9].
We categorized “early” colon or rectal cancer recurrences as occurring within five years of cancer diagnosis. “Late” colon or rectal cancer recurrence was defined as occurring more than five years after the cancer diagnosis.
Statistical analysis
For both colon and rectal cancer, we calculated the frequency and proportion of recurrence for members of the cohort within categories of sex, age group, Charlson comorbidity score, stage, surgical procedure, number of lymph nodes with metastasis, calendar period of diagnosis, surgical approach, surgical urgency, and use of neoadjuvant or adjuvant therapy. We also calculated incidence rates for each of the previous categories, with the exception of neoadjuvant or adjuvant therapy use. Using a Cox proportional hazard model, we calculated crude hazard ratios (HR) and 95% confidence intervals (CI) as well as adjusted hazard ratios and confidence intervals simultaneously modeling sex, age group at surgery date, Charlson comorbidity score, tumor stage, surgical procedure, number of metastatic lymph node category, calendar period of cancer diagnosis, surgical approach, and surgical urgency for both colon and rectal cancer. The assumption of proportionality was tested by plotting the log–log functions of the estimated survival function. The separation between the curves remained parallel across analysis time, satisfying the assumption of proportionality. We also calculated the 5- and 10-year cumulative incidences of colon and rectal cancer recurrence within each of the listed categories treating death as a competing risk, as well as the proportion of late recurrences. To visualize the cumulative incidence of colon and rectal cancer recurrence, we constructed cumulative incidence curves according to sex and calendar period of diagnosis, stratified by cancer stage. Statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA). All statistical tests were two-sided.
Results
There were 34,801 patients registered in the DCCG from 1 May 2001 to 31 December 2011, including 4649 patients with metastases and 4189 with unknown cancer stage who were excluded from analyses. The total number of eligible individuals in the DCCG database cohort was 21,152, as described in ; of these, there were 5036 recurrences identified from May 2001 to December 2012. Colon cancer patients had 3198 of these recurrences while rectal cancer patients had 1838. describes general characteristics of the study population. The frequency of colon cancer was similar among males and females, and highest in individuals between ages 65–74 and those with fewer comorbidities. The frequency of rectal cancer was highest in males, individuals between ages 65–74, and those with fewer comorbidities.
Table 1. Characteristics of the Danish Colorectal Cancer Group by cancer type.
describes incidence rates and hazard ratios within stratified characteristics for colon and rectal cancer recurrence. Colon cancer recurrence rates were highest in patients <55 years old (7.2 per 100 person-years; 95% CI: 6.5–7.9) and lowest among those ≥85 years old (5.0 per 100 person-years; 95% CI: 4.3–5.8) and were highest in patients with regional spread to at least seven lymph nodes (22 per 100 person-years; 95% CI: 20–24). Adjusted hazard ratios indicated that patients with stage III cancer (HR: 5.70; 95% CI: 4.61–7.06, compared with stage I) or patients with acute surgical urgency (HR: 1.89; 95% CI: 1.65–2.17, compared with elective surgeries) had a higher rate of colon cancer recurrence. Patients diagnosed between 2009 and 2011 (HR: 0.79; 95% CI: 0.68–0.91, compared with 2001?2004) had a decreased rate of recurrence.
Table 2. Incidence rates and hazard ratios for selected characteristics of colon and rectal cancer patients.
Like colon cancer, rectal cancer recurrence rates were highest in patients <55 years old (8.1 per 100 person-years; 95% CI: 7.2–9.0) and decreased with increasing age. Adjusted hazard ratios indicated that males (HR: 1.13; 95% CI: 1.01–1.27, compared to females), patients with stage III cancer (HR: 7.02; 95% CI: 5.58–8.82, compared with stage I) or patients with acute surgical urgency (HR: 1.85; 95% CI: 1.07–3.20, compared with elective surgeries) had a higher rate of rectal cancer recurrence. Crude hazard ratios were not appreciably different within either cancer type for most descriptive characteristic categories.
shows the 5- and 10-year cumulative incidences of recurrence and the proportion of late recurrences for colon and rectal cancer. Though males had higher 5- and 10-year cumulative incidences of colon cancer recurrence (five-year: 26%; 95% CI: 25–27%, 10-year: 30%; 95% CI: 28–31%) than females (five-year: 23%; 95% CI: 22–24%, 10-year: 27%; 95% CI: 25–28%), females had a slightly higher proportion of late colon cancer recurrences (6.0% vs. 5.5%). Patients diagnosed with stage III colon cancer (five-year: 36%; 95% CI: 35–38%, 10-year: 40%; 95% CI: 38–41%) and patients who underwent laparotomies (five-year: 26%; 95% CI: 25–26%, 10-year: 29%; 95% CI: 28–30%) had the highest 5- and 10-year cumulative incidences of colon cancer recurrence when compared with other categories within the same strata. A higher proportion of late colon cancer recurrences occurred in patients who specifically underwent resection of the transverse or sigmoid colon (Supplementary Table 4).
Table 3. Cumulative incidence and proportions of late recurrences of colon and rectal cancer patients.
Compared to females (five-year: 25%; 95% CI: 23–26%, 10-year: 29%; 95% CI: 27–31%), males had slightly higher 5- and 10-year cumulative incidences of rectal cancer recurrence (five-year: 27%; 95% CI: 26–28%, 10-year: 32%; 95% CI: 30–33%). Patients diagnosed with stage III rectal cancer (five-year: 41%; 95% CI: 39–43%, 10-year: 45%; 95% CI: 43–47%) and patients who underwent conventional abdominoperineal rectal resections (five-year: 30%; 95% CI: 27–32%, 10-year: 35%; 95% CI: 32–38%) had the highest 5- and 10-year cumulative incidences of rectal cancer recurrence when compared with other categories within the same strata (Supplementary Table 4).
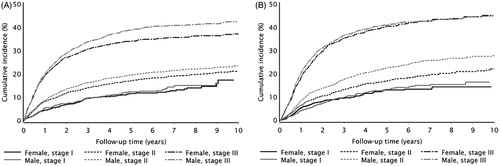
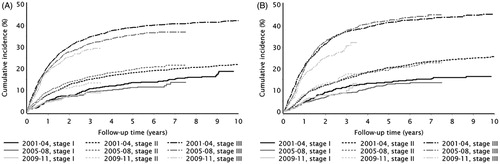
Cumulative incidence curves stratified by tumor stage show that males had a slightly higher cumulative incidence of recurrence than females over the 10-year follow-up period for stages I–III colon and rectal cancer (). shows that patients with stages I and II colon or rectal cancers had almost the same incidence of recurrence when comparing time periods of initial cancer diagnosis, with slight decreases in the cumulative incidence of colon cancer recurrence for the 2009–2011 period. However, patients diagnosed with stage III colon or rectal cancer in the period 2009–2011 had a lower incidence of recurrence after one year of follow-up than those diagnosed with stage III cancer in the years before. Both figures show that the greatest increases in recurrence occurred within three years of follow-up; the largest increase in recurrence risk occurred between the first and second year after cancer diagnosis (Supplementary Table 5).
Discussion
Although many studies describe incidence and survival rates for colorectal cancer, none has systematically measured colon or rectal cancer recurrence as an outcome, overall or with respect to descriptive characteristics. In general, the cumulative incidence of colon cancer recurrence was similar to the cumulative incidence of rectal cancer recurrence for most descriptive characteristics. In our study, age was associated with colon and rectal recurrence risk; patients who were <55 years old at initial surgery date were more likely to have a recurrence. For patients <55 years old, a higher risk of recurrence may be due in part to hereditary cancers or these individuals having more aggressive or advanced cancers than their older counterparts [Citation19,Citation20]. More studies, perhaps using pathology data, are warranted to further elucidate this pattern of recurrence. An initial diagnosis of stage III colorectal cancer, in which there is metastasis to regional lymph nodes, was strongly associated with recurrence. This risk factor is unsurprising, because tumor stage and lymph node involvement are used as morphological indicators of prognosis [Citation21]. Our results showed that increasing lymph node involvement was similarly predictive of both colon and rectal cancer recurrence.
In our crude model, patients who initially underwent laparoscopic surgery had a decreased risk of recurrence compared with patients who underwent laparotomies; however, this association was attenuated in our adjusted model. Recent studies, including randomized controlled trials and a large cohort study examining outcomes of laparoscopic surgery for colon cancer, also demonstrated and observed similar recurrence rates between the two techniques [Citation22–24]. Because laparoscopic procedures are more technically complex and not recommended for certain tumor locations (notably, obstructive right-sided or transverse colorectal cancers) [Citation25], laparotomies are still the more common procedure.
We used cumulative incidence plots to estimate absolute differences in colon and rectal cancer recurrences over time. Each of these curves showed that the greatest recurrence hazard occurred within the first three years of follow-up, and are supported by other studies showing that recurrence is most likely to occur within the first two years of an intended curative resection [Citation26–28]. The proportion of late recurrences was less than 10% for most descriptive categories of interest. These findings support current Danish guidelines for postoperative surveillance in colorectal cancer patients recommending CT scans after one and three years, and a colonoscopy every five years until the age of 75. Our tables depicting the cumulative incidences of colon and rectal cancer recurrence showed that the cumulative incidence of recurrence was similar for both cancer types, whereas previous studies have suggested that rectal cancer recurrence was higher [Citation29,Citation30]. These differences, as well as the figure illustrating lower cumulative incidences of recurrence for colon and rectal cancers diagnosed between 2009 and 2011, may be due to better staging and prognostic evaluations, ever-improving surgical procedures (e.g., total mesorectal excision), and adjuvant treatment (particularly for colon cancer) over time [Citation31,Citation32]. The incremental improvement in staging intervals over time may also be due to changes implemented through the 2010 Danish National Cancer Plan: to address delays in treatment, any patient suspected of having colorectal cancer received diagnostic work-up (including staging and a treatment plan) within 10 working days after referral to the department responsible for treatment [Citation33]. Our results are similar to a recent study that observed a decreased rectal cancer recurrence rate in Norwegian Colorectal Cancer Registry patients over a similar time period; the study also attributed these improvements to the implementation of high-quality surgical techniques and an emphasis on better treatment planning [Citation34].
One limitation to our study is that the dataset does not specify individuals whose laparoscopies were converted to laparotomies. We also lack data on detailed tumor histopathology, as histological classification of colon and rectal tumors is also a marker of disease prognosis. These types of information may have influenced treatment decision-making, so partly explain the patterns and associations between recurrence and surgery types and other treatments. Another limitation is possible misclassification of comorbidities or surgical procedures due to inaccurate coding within the databases. However, the positive-predictive value of initial cancer diagnosis in the DCR is between 95 and 98% [Citation35] and the performance characteristics of our algorithm to ascertain recurrences is also quite good [Citation9]. The strengths of our study include a population-based design with high-quality clinical registry data and complete follow-up. In addition, this is also the first population-based study to address potential risk factors for colon or rectal cancer recurrence up to 10 years after cancer diagnosis. Our ability to link population-wide information across several data sources allows for more generalizable interpretations regarding factors that may affect recurrence risk, particularly as oncology care is considered to be fairly uniform due to nationalized healthcare in Denmark [Citation1,Citation33]. Without systematic registry data, research on recurrence is limited to clinical trial settings, patient self-reporting, and small cancer cohorts with active surveillance, each of which have substantial limitations [Citation36–38].
Among colorectal cancer patients, recurrence is a common event. In our population, nearly a quarter of patients diagnosed with colon or rectal cancer had a recurrence during the 10-year follow-up period. Though there are several registries that record colorectal cancer recurrence, they are often incomplete [Citation39,Citation40]. In addition, data on factors that influence recurrence are sparse. Here, we used a validated algorithm on population-based registry data to report on several descriptive characteristics of colon and rectal cancer recurrence. Regardless of cancer type, the patterns of colon and rectal cancer recurrence observed within the first 5 years of follow-up among younger patients, patients with higher AJCC tumor stage, and patients with an increasing number of metastatic lymph nodes point to the necessity of stratifying recurrence risk so that clinicians can better plan the intensity, frequency, and duration of postoperative treatment and surveillance for individual patients. As a whole, our findings support the current Danish guidelines for postoperative surveillance in colorectal cancer patients.
IONC_1304650_Supplemental_tables.zip
Download Zip (65 KB)Acknowledgments
The authors thank all patients in the Danish Colorectal Cancer Group Database.
Disclosure statement
None declared. All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
The funding source had no role in the design, conduct, interpretation, or presentation of the research findings.
Additional information
Funding
References
- Kraftplan II. Sundhedsstyrelsen reference information. [cited 2015 Apr 3]. Available from: http://www.sst.dk/publ/publ2005/plan/kraeftplan2/Kraeftepidemiologi_rapport.pdf
- Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975-2011. National Cancer Institute. [cited 2015 Apr 3]. Available from: http://seer.cancer.gov/csr/1975_2011/ (based on November 2013 SEER data submission, posted to the SEER web site).
- American Cancer Society. Colorectal cancer facts & figures 2014-2016. Atlanta: American Cancer Society; 2014.
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–1403.
- Böckelman C, Engelmann BE, Kaprio T, et al. Risk of recurrence in patients with colon cancer stage II and III: a systematic review and meta-analysis of recent literature. Acta Oncol. 2015;54:5–16.
- Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–117.
- Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337.
- Matanoski G, Tao X, Almon L, et al. Demographics and tumor characteristics of colorectal cancers in the United States, 1998–2001. Cancer. 2006;107:1112–1120.
- Lash TL, Riis AH, Ostenfeld EB, et al. A validated algorithm to ascertain colorectal cancer recurrence using registry resources in Denmark. Int J Cancer. 2015;136:2210–2215.
- Harling H, Nickelsen T. The Danish Colorectal Cancer Database. Ugeskr Laeger. 2005;167:4187–4189.
- Nickelsen TN, Harling H, Kronborg O, et al. The completeness and quality of the Danish Colorectal Cancer clinical database on colorectal cancer. Ugeskr Laeger. 2004;166:3092–3095.
- Gogenur I, Ingeholm P, Iversen LH. [Danish Colorectal Cancer Database]. Ugeskr Laeg. 2012;174:2525.
- Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39:30–33.
- Lund JL, Frøslev T, Deleuran T, et al. Validity of the Danish National Registry of Patients for chemotherapy reporting among colorectal cancer patients is high. Clin Epidemiol. 2013;5:327–334.
- Gjerstorff ML. The Danish Cancer Registry. Scand J Public Health. 2011;39(Suppl 7):42–45.
- Erichsen R, Lash TL, Hamilton-Dutoit S, et al. Existing data sources for clinical epidemiology: the Danish National Pathology Registry and Data Bank. Clin Epidemiol. 2010;2:51–56.
- Edge SB, Byrd DR, Compton CC, et al., editors. AJCC cancer staging manual. 7th ed. New York, NY: Springer; 2010.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383.
- Kanwar SS, Poolla A, Majumdar AP. Regulation of colon cancer recurrence and development of therapeutic strategies. WJGP. 2012;3:1–9.
- Aghili M, Izadi S, Madani H, et al. Clinical and pathological evaluation of patients with early and late recurrence of colorectal cancer. Asia Pac J Clin Oncol. 2010;6:35–41.
- Zlobec I, Lugli A. Prognostic and predictive factors in colorectal cancer. J Clin Pathol. 2008; 61:561–569.
- Buunen M, Veldkamp R, Hop WC, Colon Cancer Laparoscopic or Open Resection Study Group, et al. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol. 2009;10:44–52.
- Bonjer HJ, Deijen CL, Abis GA, COLOR II Study Group, et al. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med. 2015;372:1324–1332.
- Stormark K, Søreide K, Søreide JA, et al. Nationwide implementation of laparoscopic surgery for colon cancer: short-term outcomes and long-term survival in a population-based cohort. Surg Endosc. 2016;30:4853–4864.
- Zerey M, Hawver LM, Awad Z, Members of the SAGES Guidelines Committee, et al. SAGES evidence-based guidelines for the laparoscopic resection of curable colon and rectal cancer. Surg Endosc. 2013;27:1–10.
- Waldron RP, Donovan IA. Clinical follow-up and treatment of locally recurrent colorectal cancer. Dis Colon Rectum. 1987;30:428–430.
- Obrand DI, Gordon PH. Incidence and patterns of recurrence following curative resection for colorectal carcinoma. Dis Colon Rectum. 1997;40:15–24.
- Goldberg RM. Intensive surveillance after stage II or III colorectal cancer: is it worth it? J Clin Oncol. 2006;24:330–331.
- Hellinger MD, Santiago CA. Reoperation for recurrent colorectal cancer. Clin Colon Rectal Surg. 2006;19:228–236.
- Shumate CR, Rich TA, Skibber JM, et al. Preoperative chemotherapy and radiation therapy for locally advanced primary and recurrent rectal carcinoma. A report of surgical morbidity. Cancer. 1993;71:3690–3696.
- Quirke P, Durdey P, Dixon MF, et al. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet. 1986;2:996–999.
- Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–646.
- National Cancer Plan, Copenhagen, Denmark: National Board of Health; 2010. [cited 2016 Sep 28]. Available from: http://www.epaac.eu/from_heidi_wiki/Denmark_National_Cancer_Plan_III_Danish.pdf
- Guren MG, Kørner H, Pfeffer F, et al. Nationwide improvement of rectal cancer treatment outcomes in Norway, 1993-2010. Acta Oncol. 2015;54:1714–1722.
- Storm HH, Michelsen EV, Clemmensen IH, et al. The Danish Cancer Registry-history, content, quality and use. Dan Med Bull. 1997;44:535–539.
- Ford JG, Howerton MW, Lai GY, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008;112:228–242.
- Bonevski B, Randell M, Paul C, et al. Reaching the hard-to-reach: a systematic review of strategies for improving health and medical research with socially disadvantaged groups. BMC Med Res Methodol. 2014;14:42.
- Barisic A, Glendon G, Weerasooriya N, et al. Accuracy of self-reported breast cancer information among women from the Ontario site of the Breast Cancer Family Registry. J Cancer Epidemiol. 2012;2012:310804.
- Vukasin P, Ortega AE, Greene FL, et al. Wound recurrence following laparoscopic colon cancer resection. Results of the American Society of Colon and Rectal Surgeons Laparoscopic Registry. Dis Colon Rectum. 1996;39(Suppl 10):20–23.
- Hansen MH, Balteskard L, Dorum LM, et al. Locally recurrent rectal cancer in Norway. Br J Surg. 2009;96:1176–1182.