Abstract
Introduction: Lobectomy is the standard curative treatment for non-small cell carcinoma (NSCLC) of the lung. Most studies on lobectomy have focused on short-term outcome and 30-day mortality. The aim of this study was to determine both short-term and long-term surgical outcome in all patients who underwent lobectomy for NSCLC in Iceland over a 24-year period.
Material and methods: The study involved 489 consecutive patients with NSCLC who underwent lobectomy with curative intent in Iceland, 1991–2014. Patient demographics, pTNM stage, rate of perioperative complications, and 30-day mortality were registered. Overall survival was analyzed with the Kaplan?Meier method. The Cox proportional hazards model was used to evaluate factors that were prognostic of overall mortality. To study trends in survival, the study period was divided into six 4-year periods. The median follow-up time was 42 months and no patients were lost to follow-up.
Results: The average age of the patients was 67 years and 53.8% were female. The pTNM disease stage was IA in 148 patients (30.0%), IB in 125 patients (25.4%), IIA in 96 patients (19.5%), and IIB in 50 patients (10.1%), but 74 (15.0%) were found to be stage IIIA, most often diagnosed perioperatively. The total rate of major complications was 4.7%. Thirty-day mortality was 0.6% (three patients). One- and 5-year overall survival was 85.0% and 49.2%, respectively, with 3-year survival improving from 48.3% to 72.8% between the periods 1991–1994 and 2011–2014 (p = .0004). Advanced TNM stage and age were independent negative prognostic factors for all-cause mortality, and later calendar year and free surgical margins were independent predictors of improved survival.
Conclusions: The short-term outcome of lobectomy for NSCLC in this population-based study was excellent, as reflected in the low 30-day mortality and low rate of major complications. The long-term survival was acceptable and the overall 3-year survival had improved significantly during the study period.
Introduction
Lung cancer (LC) is the leading cause of cancer-related deaths in the western world, including Iceland [Citation1,Citation2], accounting for around 26% of cancer-related deaths globally [Citation1]. In Europe and North America, non-small cell lung carcinoma (NSCLC) accounts for around 80% of LC [Citation3], where surgical resection is the only well-defined and well-studied curative treatment [Citation4]. Pulmonary resection is indicated for patients with localized or regional disease, which currently applies to almost one-third of all patients diagnosed with NSCLC in Iceland [Citation2]. Five-year survival for resected patients is reported to range from 40% to 70%, depending mostly on the TNM stage at diagnosis [Citation5,Citation6], as compared to less than 5% for non-resected patients with metastasized disease [Citation6]. Thus, resection should be offered to all patients where surgery is indicated; that is, for patients at stages I and II [Citation4] and in selected patients who are diagnosed at stage IIIA [Citation7].
Lobectomy is considered the gold standard of treatment for NSCLC [Citation4], as numerous studies have shown that lobectomy has better outcome than sublobar resections with regard to both recurrence of cancer and long-term survival [Citation8,Citation9]. Until recently, segmentectomy and wedge resection have been used when pulmonary function or other comorbidities pose a high operative risk. However, the use of segmentectomy has recently been recommended for small peripheral stage I NSCLCs instead of lobectomy [Citation10,Citation11], as outcomes are comparable to those after lobectomy. On the other hand, pneumonectomy is still reserved for more extensive disease, as the surgical morbidity and mortality are higher than for lobectomy or sublobar resections [Citation7].
Most studies on pulmonary resections for NSCLC – and especially lobectomy – have focused more on short-term outcome than on long-term survival [Citation12,Citation13]. These studies have usually been based on single tertiary-care centers, with the risk of selection bias. With lobectomy being the most common surgical procedure for NSCLC and regarded as the gold standard for curative treatment of NSCLC, this is a subgroup that is important to focus on and report their outcome. We performed a whole-nation study in Iceland, taking advantage of centralized population-based registries on the diagnosis and treatment of NSCLC, with complete long-term survival data. The main aim was to investigate both short-term and long-term outcome in NSCLC patients who underwent lobectomy in Iceland over a 24-year period, concentrating especially on 30-day and long-term survival.
Material and methods
This was a retrospective study of all patients who underwent lobectomy for primary NSCLC with curative intent in Iceland from January 1, 1991 until December 31, 2014. We excluded patients who underwent exploratory thoracotomy, exploratory video-assisted thoracoscopic surgery (VATS), pulmonary metastasectomy, palliative procedure, or resection for biopsy only. This also applied to patients with advanced disease who underwent resection without curative intent. Patients were also excluded when the postoperative pathological diagnosis showed only carcinoma in situ or one of the following: adenoid cystic carcinoma, mucoepidermoid carcinoma, carcinoid, or sarcoma. Finally, 17 patients who were at stage IV, including those with solitary metastasis to the brain or one adrenal gland at diagnosis, were excluded.
Cases were identified from a centralized database covering all the surgical specimens of LC (in Iceland) at the Department of Pathology, Landspitali University Hospital in Reykjavik. Cases identified were cross-matched with two other independent databases: a diagnosis registry and an operation registry at Landspitali University Hospital. This was done to minimize the risk of missing cases that were operated for NSCLC.
During the study period, 2556 patients were diagnosed with NSCLC in Iceland. Of them, 653 cases were surgical candidates, evaluated to tolerate a pulmonary resection. In 493 cases (75.5%), a lobectomy was performed. This included 11 patients (2.2%) who underwent lobectomy and an ipsilateral sublobar resection during the same operation and four patients (0.8%) who had a bilobectomy. The other surgical resections for NSCLC were 83 sublobar resections (wedge or segment resections; 12.7%) and 77 pneumonectomies (11.8%).
Medical records and surgical reports were reviewed and variables were registered using a standard data sheet. For each patient, over 80 different variables were collected. These included the following: age, gender, smoking habits, comorbidity (e.g., chronic obstructive pulmonary disease (COPD), ischemic heart disease (IHD), and arrhythmias), pulmonary function test results (forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), and FEV1/FVC), details of the operation, length of stay in the intensive care unit (ICU), in-hospital stay in days, pTNM stage, postoperative complications, and American Society of Anesthesiology (ASA) score, and also date and cause of death in patients who died during the follow-up period.
Preoperatively, a multidisciplinary tumor board including thoracic surgeons, pulmonologists, oncologists, radiologists, and pathologists reviewed the cases. The preoperative work-up varied between patients but usually included a chest radiograph (CXR) and computed tomography (CT) of the chest, abdomen, and head in addition to bone scintigraphy and pulmonary function tests. Positron emission tomography (PET) scan was not available in Iceland during the study period, but it was done in selected cases at Rigshospitalet, Copenhagen, Denmark. Tumor biopsies were obtained through bronchoscopy or transthoracic CT-guided needle biopsy. Mediastinoscopy was done routinely since 2005 and endoscopic bronchial ultrasound (EBUS) with transbronchial needle aspiration biopsies was performed in a proportion of cases since 2013. The seventh edition of the TNM staging system was used to stage patients postoperatively (pTNM). Detailed clinical TNM (cTNM) staging was not adequately registered for all patients, so only pathological staging (pTNM) is presented in this study.
Landspitali University Hospital is the only hospital in Iceland in which cardiothoracic surgery is performed, and this study was therefore done on a nationwide basis. All the surgical procedures were performed by nine surgeons, one of whom performed the vast majority of them in the last decade. All the patients had epidural placed for postoperative analgesia and general anesthesia with double lumen endotracheal tube for lung isolation. Posterolateral thoracotomy was the method used during more than half of the study period, but during the last 10 years, an anterolateral approach was the preferred method. During the study period, video-assisted thoracoscopic surgery (VATS) was not used for lobectomies but it was used for selected wedge resections and segmentectomies. The lobectomies were standardized with intraoperative lymphadenectomy of enlarged hilar or ipsilateral mediastinal lymph nodes, and from 2005 with routine ipsilateral mediastinal lymphadenectomy according to the ESTS guidelines [Citation14,Citation15].
Ex-smokers were defined as patients who had stopped smoking more than 5 years before surgery, and never-smokers were defined as patients who had smoked less than 100 cigarettes in their lifetime. Surgical complications were divided into major and minor complications. Major complications were defined as: bronchopleural fistula (BPF), myocardial infarction (MI), adult respiratory distress syndrome (ARDS), reoperation for postoperative bleeding, congestive heart failure (CHF), and empyema with or without reoperation. Minor complications were defined as: new-onset atrial fibrillation, postoperative pneumonia, recurrent nerve paralysis, wound infection, air leakage over 7 days, and intraoperative bleeding of >1 L (without reoperation). Operative mortality was defined as death within 30 days of surgery, but hospital mortality and 90-day mortality were also registered. Patients were assigned a date of death or identified as living on September 1, 2016, using data from the Icelandic National Population Registry. Median follow-up time was 42 months (mean 62.6 months, range: 1–279).
To investigate trends in survival, the 24-year study period was divided up into six 4-year periods.
Statistics
Microsoft Excel 2010 was used for descriptive statistics and R version 3.1.3 (Wien, Austria) was used for survival analysis. Student’s t-test and ANOVA were used to compare continuous variables between groups of two or more, following use of the Kolmogorov–Smirnov test (KS test) for assessment of normality of the data. Chi-square test was used to compare categorical variables and Fisher’s exact test was used if the values had an expected frequency of 10 or less. Differences were considered to be significant when the p value was less than 0.05. The Kaplan–Meier method was used to calculate overall survival (OS) and log-rank test was used to compare survival between groups. In order to identify factors that were prognostic of long-term survival, the Cox proportional-hazards model was used. Factors that had a p value of less than 0.1 in univariate analysis were used in the preliminary model along with factors that have been shown to be significant in other studies. A subset of variables was chosen for inclusion in the final model, using a stepwise selection procedure. To check our assumption of proportionality, a global goodness-of-fit test was done together with graphic plotting of variables.
The study was approved by the Icelandic National Bioethics Committee (reference number: 98-060-CM) and the Data Protection Authority (reference number: 2001011025SJ/eb). As individual patients were not identified, the need for individual consent was waived.
Results
Patient demographics and cardiovascular risk factors are shown in . Of the 493 lobectomies for NSCLC, 265 (53.8%) were performed on female patients. The mean age was 67 ± 9.5 years, and similar for both genders (p = .81). More women had smoked within 5 years of surgery (p = .0007), but more men had a history of ischemic heart disease (p < .0001). More women had adenocarcinoma histology: 74.3% as compared to 59.6% in men (p = .0004). On the other hand, no statistically significant gender differences were found for history of COPD, arrhythmias, preoperative FEV1 < 75%, or ASA score. Altogether, 184 patients (37.3%) were diagnosed incidentally, most often due to diseases or symptoms unrelated to NSCLC that had resulted in a chest X-ray or CT-scan. In the other 309 cases, the patients had symptoms of LC, and cough (36.3%), dyspnea (23.1%), chest pain (17.2%), pneumonia (17.4%), and/or weight loss (15.8%) were the most common symptoms. Other symptoms such as fever (11.4%) and hemoptysis (7.3%) were less common.
Table 1. Patient demographics, risk factors, comorbidities, complications, tumor factors, and staging for 493 cases that underwent a lobectomy for NSCLC in Iceland, 1991–2014. The table also shows univariate association of the patient demographics and comorbidities with long-term survival.
The mean operative time was 136 min (range: 30–395 min) and median hospital stay was 9 days (range: 2–144 days). The most common histological types were adenocarcinoma (65.3%) and squamous cell carcinoma (25.8%), but large-cell and adenosquamous carcinomas were less common (5.9% and 3.0%, respectively). In 460 of the cases (93.3%), the patients had cancer-free surgical margins; the remaining 33 patients had microscopic disease at the resection margins (positive margins). The mean size of the tumors was 3.6 ± 2.3 cm, ranging from 0.2 to 19.5 cm.
Postoperative TNM staging is shown in . More than half of the patients had stage I disease (55.6%), most of them being stage IA (30.0% of the whole study population). Patients at stage II were 29.6%, with 19.5% being at stage IIA and 10.1% at stage IIB. A further 74 patients (15.0%) had surgically resectable locally advanced disease (stage IIIA), most of it being diagnosed perioperatively – with microscopic N2 ipsilateral mediastinal lymph node involvement or T4 tumor with local invasion to the mediastinum. shows that the proportion of patients diagnosed at stage IA increased from 25.9% in 1991–1994 to 39.4% in 2011–2014 (p = .03). No significant changes between time periods were found for other stages (IB, IIA, IIB, and IIIA). Furthermore, the mean age of patients did increase during the study period, from 63.2 years in 1991–1994 to 67.6 years in 2011–2014 (p = .009), and the 30-day mortality did not change significantly between the 4-year time periods (p = .23).
Table 2. Stage distribution of 493 cases operated with lobectomy for NSCLC in Iceland over 24 years, divided into 4-year periods, expressed as n (% per period).
Both minor and major complications are listed in , but some patients had several major and/or minor complications. In 104 cases (21.1%), the patient suffered one or more complications, with 23 patients (4.7%) having at least one major complication and 81 patients (16.4%) having at least one minor complication. The most common complication was persistent chest tube air leakage for more than 7 days (17.2%), followed by pneumonia (8.1%) and intraoperative bleeding exceeding 1 L (7.1%).
The 30-day mortality was 0.6% (three patients), hospital mortality was 1.2% (six patients), and 90-day mortality was 1.4% (seven patients). Two of the three patients who did not survive for 30 days died on postoperative day 11 – one from pneumonia, sepsis, and hypotension and the other from cardiogenic shock related to myocardial infarction. The third patient had respiratory failure due to pneumonia that required a tracheostomy and ventilator therapy, but eventually died on postoperative day 23. Patients who died in hospital after postoperative day 30 usually died of respiratory failure, but they all had compromised pulmonary function preoperatively.
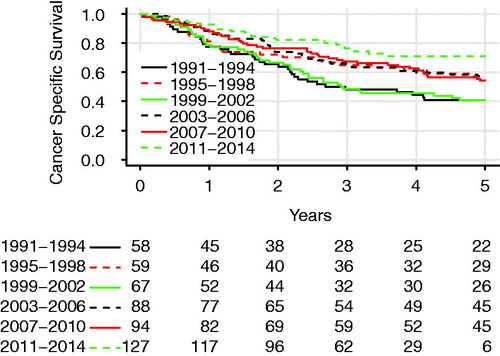
OS for all patients is plotted in , with the Kaplan–Meier graph showing 85.0%, 60.9%, and 49.2% of the patients being alive at 1, 3, and 5 years postoperatively. shows the OS in different 4-year time periods. CSS is shown in and was estimated to be 85.9%, 63.8%, and 54.6% at 1, 3, and 5 years, respectively. Both OS and CSS improved significantly during the study period, with 3-year OS increasing from 48.3% in 1991–1994 to 72.8% in 2011–2014 (log-rank test, p = .0004). The 3-year CSS was 48.3% in 1991–1994 and 76.6% in 2011–2014 (log-rank test, p < .001). The 5-year OS was 64.2% for stage I, 35.1% for stage II, and 20.4% for stage IIIA.
Figure 1. Overall survival of all patients who underwent lobectomy for NSCLC with curative intent in Iceland, 1991–2014. The broken line represents the 95% confidence interval and crosses indicate censored cases.
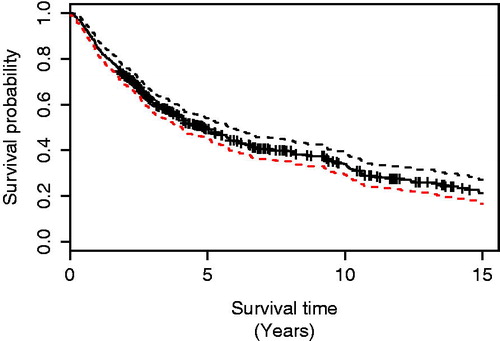
Figure 2. Overall survival of patients who had a lobectomy with curative intent for NSCLC in Iceland in different 4-year time periods. The difference was significant (log-rank test, p = .003).
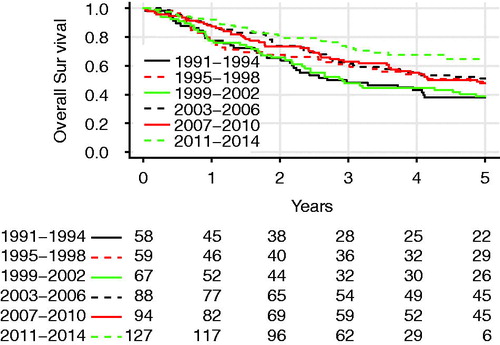
Figure 3. Comparison of cancer-specific survival in patients operated with lobectomy for NSCLC in Iceland in different 4-year time periods. The difference was significant (log-rank test, p < .001).
shows a univariate analysis of the predictors for OS. The predictors of increased mortality were advanced age (HR = 1.03 per year, 95% CI: 1.02–1.05; p = .001), ischemic heart disease (HR = 1.47, 95% CI: 1.15–1.88; p = .002), any major complications (HR = 1.69, 95% CI: 1.07–2.66; p = .02), postoperative congestive heart failure (HR = 2.18, 95% CI: 1.16–4.1; p = .01), postoperative pneumonia (HR = 1.58, 95% CI: 1.08–2.31; p = .02), squamous cell histology (HR = 1.28, 95% CI: 1.01–1.63; p = .04), and advanced stage (HR = 3.62 for stage IIIA compared to stage IA, 95% CI: 2.56–5.14; p < .0001), which was by far the strongest prognostic factor. Protective factors regarding mortality were female gender (HR = 0.72, 95% CI: 0.57–0.89; p = .003), free surgical margins (HR = 0.33, 95% CI: 0.23–0.5; p < .0001), and later calendar year of treatment (HR for the period 2011–2014 was 0.45, 95% CI: 0.49–0.69; p < .0001).
Independent negative factors regarding survival were advanced age (HR = 1.03, 95% CI: 1.02–1.04; p < .0001) and advanced stage (HR = 1.4, 95% CI: 1.25–1.46; p < .0001). After correction for stage and other significant factors in the multivariate model (see ), later calendar year turned out to be a significant protective factor regarding mortality, with an HR of 0.88 per year (95% CI: 0.82–0.94; p = .0003). Furthermore, a cancer-free surgical margin was also found to be an independent factor prognostic of lower mortality (HR = 0.44, 95% CI: 0.30–0.65; p < .0001). The patients who did not have free surgical margins usually received adjuvant chemotherapy, and were not reoperated or did not receive adjuvant radiation.
Table 3. Cox multivariate analysis for all patients who underwent lobectomy for NSCLC in Iceland, 1991–2014.
Discussion
In this whole-nation study, we investigated both short-term and long-term outcome after lobectomy for NSCLC over a 24-year period in a well-defined cohort of Icelandic patients. The 30-day mortality was very low – only 0.6% – with almost every second patient (49.2%) surviving for at least 5 years. Furthermore, the 3-year OS improved significantly with time: from 48.3% in 1991–1994 to 72.8% in 2011–2014 (p = .0004).
The explanation for the significant increase in OS seen during the last 4-year period is probably multifactorial. However, one of the main influential factors may have been the increased ratio of patients with stage IA disease, which rose from 25.9% to 39.4% between the periods 1991–1994 and 2011–2014 (p = .03). The reason for this increase in early-stage NSCLC is unclear, but in Iceland, no screening for lung cancer has been implemented and our analysis did not show that the proportion of tumors that were diagnosed incidentally had increased significantly during the study period (p = .47). The increase in early-stage NSCLC is more likely explained by improved imaging techniques and more thorough postoperative follow-up, as well as increased public awareness. When we corrected for stage and higher age in the multivariate analysis, later calendar year was found to be an independent factor of increased survival. The improved outcome therefore appears to be influenced by factors other than stage and incidental diagnosis. Thirty-day mortality, a surrogate marker for surgical technique, perioperative care, and postoperative complications, remained very low during the 24-year study period (p = .23) and does not therefore explain improved survival. The minor and major complication rate stayed constant during the study period (p = .70 and p = .46, respectively). The fact that nine surgeons performed the surgical procedures and one of them performed the vast majority in the last decade could have had an effect on short-term outcomes, such as complications and length of stay. Other possible factors, such as improved diagnostic work-up with increased use of CT scans may have contributed as well as more thorough preoperative staging with mediastinoscopies performed routinely since 2005. This, along with increased use of adjuvant chemotherapy, may also have contributed. Our findings are in line with a recent Norwegian study where similar findings regarding survival to those in the present study were reported for NSCLC patients [Citation16]. Unfortunately, we did not have detailed information on the use of adjuvant chemotherapy in our patients, but it has been offered to most stage II and IIIA patients since 2003. Thus, the direct effect of adjuvant chemotherapy could not be analyzed.
The factors that were prognostic of mortality in the present study were advanced pTNM stage (HR = 3.62 for stage IIIA vs. stage IA, 95% CI: 2.56–5.14; p < .0001), positive surgical margins, advanced age, and calendar year of treatment (time period). TNM stage, advanced age, and positive surgical margins have all been reported previously to be negative prognostic factors in other studies [Citation5,Citation6,Citation17]. To our knowledge, however, only very few studies have reported improved survival according to calendar year. The most important is the study by Morgant et al. [Citation18], which found better survival in lobectomy patients from 2005 to 2012 in France, and a Norwegian study by Nilssen et al. [Citation16], which found a gain in 5-year survival from 47% in the period 1997–2003 to 62.6% in 2004–2011, in all resected NSCLC patients. Furthermore, in recent unpublished studies (presented as abstracts) based on the SEER (Surveillance, Epidemiology, and End Results) database, improved survival was reported for patients with stage-II adenocarcinoma and squamous cell carcinoma from 1988 until 2013 [Citation19,Citation20].
Other factors reported to be prognostic of increased mortality in the literature are a history of weight loss and positive tumor markers (i.e., KRAS, EGFR, etc.) [Citation17]. In our retrospective study, weight loss was not a prognostic factor regarding survival and tumor markers were not routinely measured until the later part of the study.
The fact that OS for the whole patient cohort was found to be around 50% at 5 years underscores the fact that many of the patients who undergo lobectomy – even patients with localized stage I disease – later die from spread of NSCLC, a recurrent disease, or other smoking-related diseases such as ischemic heart disease or COPD. In comparable studies, the OS at 5 years usually lies between 40% and 70% [Citation9,Citation21]. The OS found in the present study is therefore in line with other reports. In the study by Morgant et al. [Citation18], which included all pulmonary resections for NSCLC in France operated between 2005 and 2012, the 3-year survival for lobectomy patients was 82%, as compared to 71% in the present study. However, the French study also included non-malignant carcinoid tumors (with typical histology) and other benign tumors in 10% of cases, where the prognosis is very favorable [Citation22] compared to other histological types of NSCLC – which all the patients in the present study were diagnosed with.
In the present study, 15% of the patients had stage IIIA disease that was usually detected postoperatively after the finding of positive ipsilateral microscopic mediastinal lymph node sampled at surgery, or a T4 tumor that had invaded the mediastinum. In a more recent study – by Dickhoff et al. [Citation13] – the 4-year survival following lobectomy ranged from 27% to 51% in the period 2010–2013. This wide difference in survival was based on different induction therapies, and is in line with our 4-year survival of 27.2% over the whole period, which had improved to 41% in the period 2011–2014. During the last period, adjuvant chemotherapy was offered to all patients at stage IIIA, as recommended by the European Society for Medical Oncology (ESMO) guidelines [Citation7]. The 3-year survival for stages II and IIIA together was 49% in the present study, as compared to 56.6% in a newly published study by Park et al. [Citation23], where all the patients received induction therapy. In our study, very few of the patients had induction therapy.
Compared to other reports, the overall rate of complications was relatively low in the present study (21.1%), with the complications usually being classified as minor (16.4%). Furthermore, the overall rate of major complications was only 4.7%, which also contributed to the very low 30-day mortality (only 0.6%; three patients). For comparison, other studies have reported an overall rate of complications in the 19.1–58.2% range, [Citation24–26] and the 30-day mortality has most often been between 1% and 2.5%. However, 30-day mortality up to 4.1% has also been reported in the literature [Citation24,Citation26,Citation27]. In the study by Irie et al., a lower 30-day mortality than ours was reported (0.5%), and there was an overall complication rate of 19.1% [Citation24]. However, that study only involved 188 patients who were operated at a single center over a 7-year period, with all of them being at cTNM stage I and being operated with VATS technique. The present study, however, covered 24 years with patients at stages I–IIIA, with all of them operated with open thoracotomy.
The most common complication in the present study was prolonged air leakage (17% of cases) – with a range of 9.6–24% in other comparable studies [Citation24,Citation28]. This difference could possibly be explained by different definitions of prolonged air leakage or different management of chest tubes between centers. Other postoperative complications in the present study were also in line with previous reports, such as pneumonia, new-onset atrial fibrillation, and perioperative myocardial infarction [Citation24,Citation29].
The major limitation of our study is its retrospective design, with the drawbacks that this can entail, such as the lack of information on symptoms, the lack of information on clinical staging, and the lack of documentation on complications. During the study period, PET scan was not available in Iceland and mediastinoscopy was only routinely used for staging of mediastinal lymph nodes during the later half of the study period. The main strength of the study, however, is the fact that the patient cohort consisted of patients representing a whole population, with all the patients being operated at a single center, reducing the risk of selection bias and institutional bias. Our single-center nationwide approach therefore eliminated referral bias and made identification of risk profiles more reliable.
Conclusions
According to this population-based study, the short-term outcome of lobectomy for NSCLC is excellent in Iceland, as reflected by a low rate of complications and a 30-day mortality of only 0.6%. Long-term overall survival, with every second patient surviving 5 or more years after surgery, was acceptable and comparable to that in other studies. Importantly, the survival improved over the 24-year study period. The explanation for this gain in survival is not obvious, but it is most likely explained by the combination of improvements in staging, postoperative care, surgical technique, and adjuvant chemotherapy for stage II/IIIA patients.
Acknowledgments
We thank the members of the Lung Cancer Research Group at Landspitali University Hospital for help with data collection, and Gunnhildur Johannsdottir for secretarial help.
Disclosure statement
The authors declare that they have no competing interests.
Additional information
Funding
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
- Krabbameinsskra [Internet]. Krabbameinsskrá Reykjavík: Krabbameinsfélagið; 2016 [cited 2016 Oktober 13]. Available from: http://www.krabbameinsskra.is
- Meza R, Meernik C, Jeon J, et al. Lung cancer incidence trends by gender, race and histology in the United States, 1973–2010. PLoS One. 2015;10:e0121323.
- Vansteenkiste J, Crino L, Dooms C, et al. 2nd ESMO Consensus Conference on Lung Cancer: early-stage non-small-cell lung cancer consensus on diagnosis, treatment and follow-up. Ann Oncol. 2014;25:1462–1474.
- Woodard GA, Jones KD, Jablons DM. Lung cancer staging and prognosis. Cancer Treat Res. 2016;170:47–75.
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11:39–51.
- Eberhardt WE, De Ruysscher D, Weder W, et al. 2nd ESMO Consensus Conference in Lung Cancer: locally advanced stage III non-small-cell lung cancer. Ann Oncol. 2015;26:1573–1588.
- Pass H, Carbone DP, Johnson DH, et al., editors. Principle and practice of lung cancer. Philadelphia (PA): Lippincott Williams & Wilkins; 2010.
- Thorsteinsson H, Alexandersson A, Oskarsdottir GN, et al. Resection rate and outcome of pulmonary resections for non-small-cell lung cancer: a nationwide study from Iceland. J Thorac Oncol. 2012;7:1164–1169.
- Yang F, Sui X, Chen X, et al. Sublobar resection versus lobectomy in Surgical Treatment of Elderly Patients with early-stage non-small cell lung cancer (STEPS): study protocol for a randomized controlled trial. Trials. 2016;17:191.
- Deng HY, Wang YC, Ni PZ, et al. Radiotherapy, lobectomy or sublobar resection? A meta-analysis of the choices for treating stage I non-small-cell lung cancer. Eur J Cardiothorac Surg. 2017;51:203–210.
- Kim ES, Kim YT, Kang CH, et al. Prevalence of and risk factors for postoperative pulmonary complications after lung cancer surgery in patients with early-stage COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:1317–1326.
- Dickhoff C, Dahele M, de Langen AJ, et al. Population-based patterns of surgical care for stage IIIA NSCLC in the Netherlands between 2010 and 2013. J Thorac Oncol. 2016;11:566–572.
- De Leyn P, Lardinois D, Van Schil PE, et al. ESTS guidelines for preoperative lymph node staging for non-small cell lung cancer. Eur J Cardiothorac Surg. 2007;32:1–8.
- De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg. 2014;45:787–798.
- Nilssen Y, Strand TE, Fjellbirkeland L, et al. Lung cancer survival in Norway, 1997–2011: from nihilism to optimism. Eur Respir J. 2016;47:275–287.
- Ost DE, Yeung SC, Tanoue LT, et al. Clinical and organizational factors in the initial evaluation of patients with lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e121S–e141S.
- Morgant MC, Pages PB, Orsini B, et al. Time trends in surgery for lung cancer in France from 2005 to 2012: a nationwide study. Eur Respir J. 2015;46:1131–1139.
- Nguyen DT, Fontaine JP, Robinson LA, et al. P1.22: Temporal survival improvement for stage-II (T3N0M0) lung adenocarcinoma after pulmonary lobectomy: track: early stage NSCLC (Stage I–III). J Thorac Oncol. 2016;11:S195.
- Nguyen DT, Fontaine JP, Robinson LA, et al. P1.17: Improved survival for stage-2 (N1) pulmonary adenocarcinoma and squamous cell carcinoma after pulmonary lobectomy: track: early stage NSCLC (Stage I–III). J Thorac Oncol. 2016;11:S191–S1S2.
- Peters S, Weder W, Dafni U, et al. Lungscape: resected non-small-cell lung cancer outcome by clinical and pathological parameters. J Thorac Oncol. 2014;9:1675–1684.
- Sigurdardottir JM, Isaksson HJ, Johannsson KB, et al. Histology does not accurately predict the clinical behaviour of bronchopulmonary carcinoids – results from an Icelandic population-based study. Laeknabladid. 2008;94:125–130.
- Park BJ, Yang HX, Woo KM, et al. Minimally invasive (robotic assisted thoracic surgery and video-assisted thoracic surgery) lobectomy for the treatment of locally advanced non-small cell lung cancer. J Thorac Dis. 2016;8:S406–S413.
- Irie M, Nakanishi R, Yasuda M, et al. Risk factors for short-term outcomes after thoracoscopic lobectomy for lung cancer. Eur Respir J. 2016;48:495–503.
- Andalib A, Ramana-Kumar AV, Bartlett G, et al. Influence of postoperative infectious complications on long-term survival of lung cancer patients: a population-based cohort study. J Thorac Oncol. 2013;8:554–561.
- Thomas PA, Berbis J, Falcoz PE, et al. National perioperative outcomes of pulmonary lobectomy for cancer: the influence of nutritional status. Eur J Cardiothorac Surg. 2014;45:652–659. discussion 9.
- Launer H, Nguyen DV, Cooke DT. National perioperative outcomes of pulmonary lobectomy for cancer in the obese patient: a propensity score matched analysis. J Thorac Cardiovasc Surg. 2013;145:1312–1318.
- Stolz AJ, Schutzner J, Lischke R, et al. Predictors of prolonged air leak following pulmonary lobectomy. Eur J Cardiothorac Surg. 2005;27:334–336.
- Berry MF, Onaitis MW, Tong BC, et al. A model for morbidity after lung resection in octogenarians. Eur J Cardiothorac Surg. 2011;39:989–994.
